FIGURE 4.
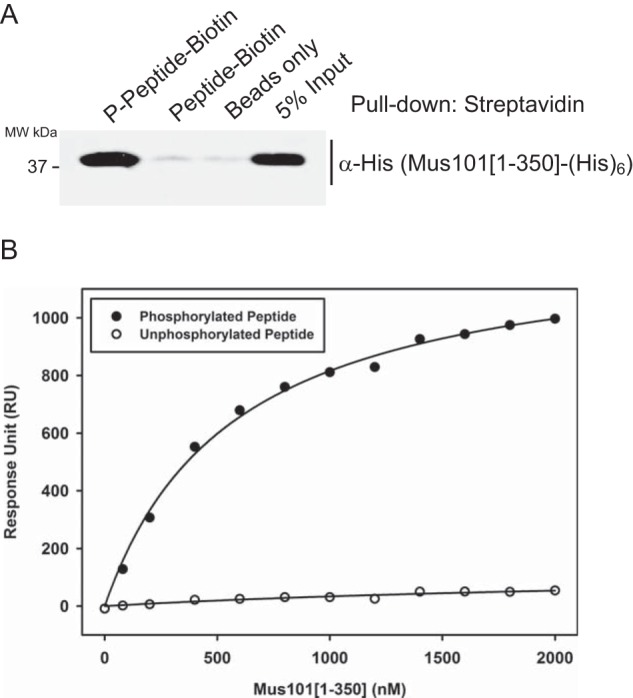
Phosphorylated Top2 peptide interacts with Mus101[1–350]-His6. C-terminal biotinylated synthetic peptides, phosphorylated (Ser(P)-1428 and Ser(P)-1443) or unphosphorylated, containing the last 25 amino acids of Top2, were examined with a pulldown assay and a surface plasmon resonance experiment. A, Mus101[1–350]-His6 was incubated with immobilized peptides on streptavidin-agarose beads in the pulldown experiment, and only phosphorylated peptide was found to have binding activity. MW, molecular weight. B, 145 response units (RU) of phosphorylated or unphosphorylated peptides were immobilized on separate channels of a streptavidin chip for a surface plasmon resonance experiment. Different concentrations of Mus101[1–350]-His6 (from 0–2 μm) were tested, and sensorgrams were recorded. Saturated response units of each condition were plotted against the concentrations of Mus101[1–350]-His6. The Kd of Mus101[1–350]-His6 to phosphorylated Top2 peptide was determined to be 0.57 ± 0.04 μm.
