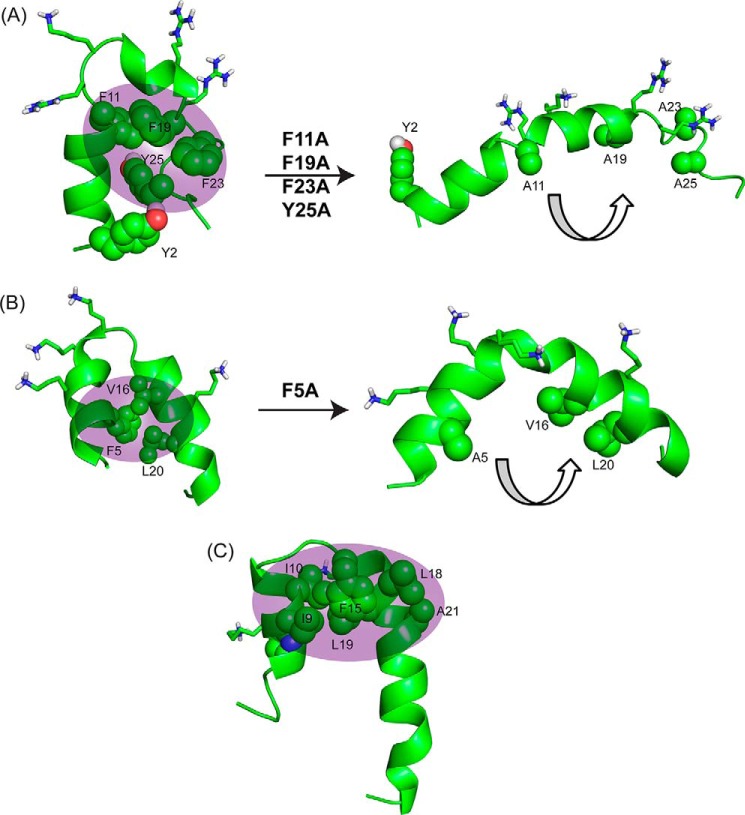
FIGURE 7.
Comparison of structural motifs of KYE28/KYE28A with MSI-594/MSI-594F5A and Pardaxin to elucidate importance of hydrophobic cluster. A, NMR derived three-dimensional structure of KYE28 and KYE28A showing the importance of the aromatic zipper (sphere representation) in stabilization of the helix-loop-helix motif; B, NMR derived three-dimensional structure of MSI-594 (PDB accession code 2K98) showing a similar helix-loop-helix motif stabilized by F5 (44). The removal of F5 (PDB accession code 2L36) (36) leads to an opened out curved helix yet again indicating the importance of the hydrophobic interactions mediated by the aromatic residue in forming the helix loop helix motif. C, NMR derived helical hairpin structure of Pardaxin in LPS (PDB accession code 2KNS). Phe15 plays an important role to stabilize the structure by connecting the two helical segments (22).