FIGURE 8.
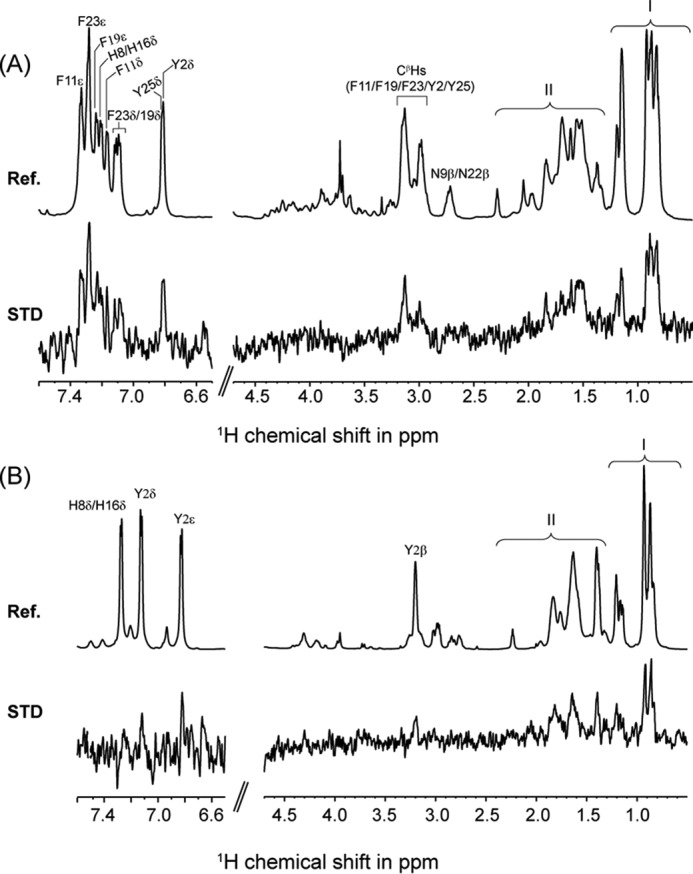
STD NMR and docking studies of interaction between KYE28/KYE28A and LPS. A, reference 1H NMR spectra and STD NMR spectra of KYE28 in LPS. The spectra show side chain resonances of aromatic amino acids Tyr2, His8, Phe11, His16, Phe19, Phe23,and Tyr25. Spectral regions indicated by I and II represent overlap in the signal intensities of the side chain resonances of Ile4, Ile7, Leu10, Leu14, Leu18, and Leu27, and positively charged residues Lys1, Arg12, Lys13, Arg20, Arg21, and Arg28, respectively. B, reference 1H NMR spectra and STD NMR spectra of KYE28A in LPS. The spectra shows side chain resonances of aromatic amino acid residue Tyr2, His8, and His16. Spectral regions indicated by I and II represent overlap in the signal intensities of the side chain resonances of Ile4, Ile7, Leu10, Ala11, Leu14, Leu18, Ala19, Ala23, Ala25, and Leu27, and positively charged residues Lys1, Arg12, Lys13, Arg20, Arg21, and Arg28, respectively.
