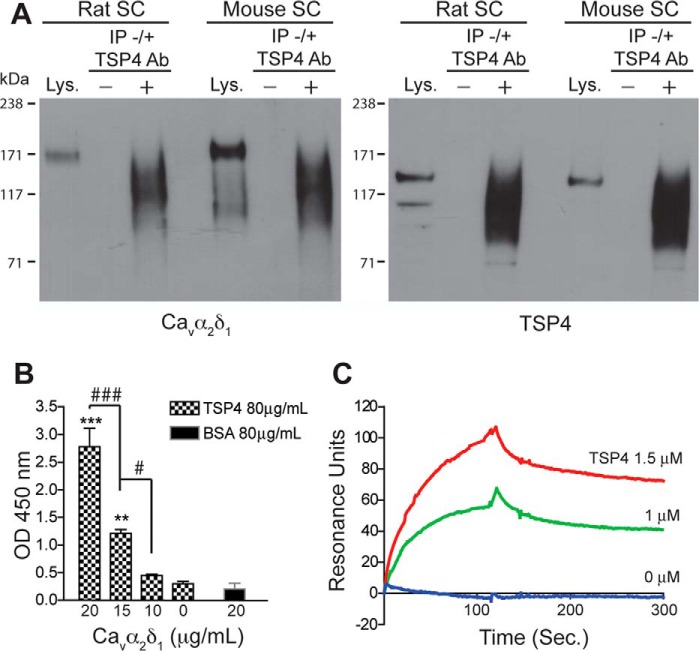
FIGURE 1.
TSP4 and Cavα2δ1 interaction. Immunoprecipitation, solid-phase binding, and surface plasmon resonance binding were performed as described under “Materials and Methods” to detect TSP4/Cavα2δ1 interaction in rodent spinal cord and in vitro. A, typical Western blots showing Cavα2δ1 co-immunoprecipitation with TSP4 proteins (IP) by anti-TSP4 antibodies from rat or mouse spinal cord (SC) samples (from n ≥ 3 each). Lys, spinal cord lysate positive control. −, no anti-TSP4 IP antibody. +, with anti-TSP4 IP antibody. Approximate positions of prestained molecular weight markers are shown on the left of each gel. B, solid phase binding showing dose-dependent FLAG-Cavα2δ1 binding to immobilized TSP4. **, p < 0.01; ***, p < 0.001 compared with no Cavα2δ1; #, p < 0.05; ###, p < 0.001 between adjacent doses by one-way ANOVA with Bonferroni post-tests. C, surface plasmon resonance binding sensogram of dose-dependent TSP4 binding to captured Cavα2δ1 (typical of three independent experiments).