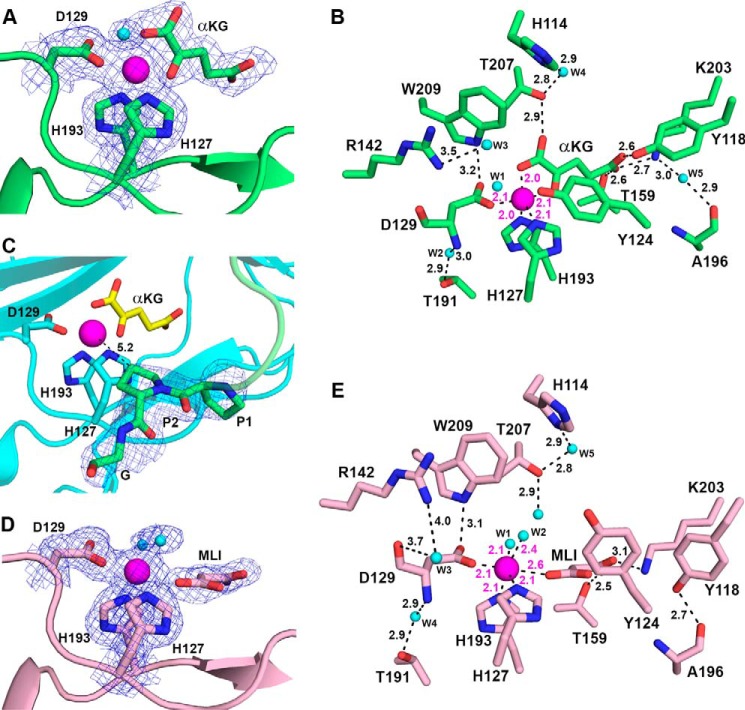
FIGURE 6.
Active site of BaP4H with bound cofactors. A, bidentate binding mode for αKG in Co(II)-BaP4H-PPG structure. B, active site residues of Co(II)-BaP4H-PPG involved in hydrogen bonding interactions between protein, αKG, and water are indicated by black dashes with corresponding distances. The distance between Tyr124 and W1 is 2.8 Å and was excluded from the picture for clarity. C, the PPG peptide (green) at the C terminus interacts with the active site of a symmetry related molecule (cyan). The Y proline (P2) is oriented with the C4 carbon 5.2 Å away from the cobalt metal center. The 2nd and 3rd repeats of (P-P-G)3 were disordered. D, monodentate binding mode for malonate in Co(II)-BaP4H-MLI structure. E, active site residues of Co(II)-BaP4H-MLI showing hydrogen bonding interactions between protein, malonate, and water with labeled distances. αKG, malonate, and protein residues are shown as sticks: carbon (protein backbone color), oxygen (red), nitrogen (blue), water molecules as spheres (cyan), cobalt as a sphere (magenta). The 2Fo − Fc composite omit maps (blue mesh) contoured at 1.0 σ are shown for protein residues in A, C, and D. The Fo − Fc omit maps (also shown as blue mesh) for αKG and MLI are contoured at 3.0 σ in A and D.