FIGURE 9.
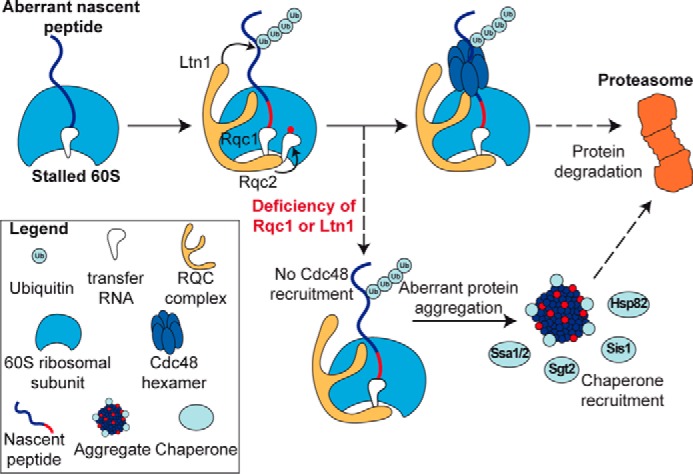
Model for the mechanism of aberrant protein aggregation, recognition, and proteasomal degradation in the absence of Rqc1 or Ltn1. Aberrant nascent peptides are recognized at the 60S level by the RQC complex, which enables its polyubiquitylation by Ltn1 and CAT-tail addition by Rqc2. Rqc1 is then essential for the recruitment of a Cdc48 hexamer to extract the peptide from the 60S exit tunnel and escort it to the proteasome for degradation. The absence of Rqc1 or Ltn1 causes a defect in Cdc48 recruitment, which results in CAT-tail-dependent protein aggregation. These cytosolic aggregates are recognized by specific co-chaperones (Sis1 and Sgt2) and chaperones (Ssa1, Ssa2, and Hsp82). Eventually, aggregated species of aberrant proteins are also eliminated by the proteasome.
