Abstract
Background:
Chrysophanic acid, also known as chrysophanol, has a number of biological activities. It enhances memory and learning abilities, raises superoxide dismutase activity, and has anti-cancer effects in several model systems. According to previous reports, chrysophanic acid-induced cell death shares features of necrotic cell death. However, the molecular and cellular processes underlying chrysophanic acid-induced cell death remain poorly understood.
Methods:
Chrysophanic acid-induced cell death was monitored by cell viability assay and Annexin V-propidium iodide (PI) staining of renal cell carcinoma Caki-2 cells. The induction of intracellular reactive oxygen species (ROS) by chrysophanic acid and the suppression of ROS by anti-oxidants were evaluated by 2′,7′-dichlorofluorescin diacetate staining. The expression and phosphorylation of proteins that are involved in apoptosis and necroptosis were detected by immunoblotting.
Results:
The extent of chrysophanic acid-induced cell death was concentration and time dependent, and dead cells mainly appeared in the PI-positive population, which is a major feature of necrosis, upon fluorescence-activated cell sorting analysis. Chrysophanic acid-induced cell death was associated with the generation of intracellular ROS, and this effect was reversed by pretreatment with N-acetyl cysteine. Chrysophanic acid-induced cell death was not associated with changes in apoptotic or necroptotic marker proteins.
Conclusions:
The cell death induced by chrysophanic acid resembled neither apoptotic nor necroptotic cell death in human renal cell carcinoma Caki-2 cells.
Keywords: Chrysophanic acid, Human renal cell carcinoma, Reactive oxygen species, Necrosis, Necroptosis
INTRODUCTION
Renal cell carcinoma (RCC) is the most common type of kidney cancer and is classified into several subtypes based on microscopic characteristics.1,2 In general, partial or complete surgical removal of the affected kidney is the only effective treatment for localized RCC.3 In 25% to 30% of patients, primary RCC metastasizes from the kidney to other organs, such as the lymph nodes, lungs, and liver.4 RCC has a poor prognosis owing to high heterogeneity, rate of metastasis, and resistance to conventional anti-cancer therapies.5 Therefore, the development of novel therapeutic agents for RCC therapy is required, and much research focuses on compounds found in natural products.
Chrysophanic acid is a compound derived from Rheum officinale or Polygpnum cuspidatum and belongs to anthraquinone family.6–8 According to previous studies, anthraquinones and their derivatives show various biological effects, including anti-cancer,6,9 anti-microbial,10 and hepatoprotective effects.11 A number of recent studies reported that chrysophanic acid induces necrosis-like cell death in several human cancer cells, namely, in lung cancer A549, liver cancer J5, and Hep3B cells.11–13 However, the mechanisms underlying the anti-cancer effect of chrysophanic acid remain to be elucidated.
Cell death is the natural process of removing abnormal cells and may result from disease, physical injury, and intrinsic or extrinsic stimuli, such as chemicals, DNA damage, and death-inducing ligands. Cell death can be classified into apoptosis, necrosis, or necroptosis according to morphological and molecular hallmarks. Apoptosis, or programmed cell death, is a biological process for eliminating unwanted cells and associated with cell shrinkage, the formation of an apoptotic body, and DNA fragmentation.14 Apoptosis is induced by two different pathways: one is the intrinsic pathway mediated by the mitochondria, the other is the death receptor (DR)-mediated extrinsic pathway.15 Necrosis is disorderly and passive cell death in response to acute and severe stress.16 Necrosis is usually followed by inflammatory reactions,17 and not associated with activation of caspase cascades.18,19 Necroptosis is a programmed form of necrosis and very common in vivo, in neurodegeneration, and in ischemia-induced cell death or cell death as a result of microbial infection. Necroptosis is quite different from uncontrolled necrosis and shares several cellular processes with apoptosis.20,21
In this study, the underlying molecular and cellular processes through which chrysophanic acid induces cell death were investigated in human renal cell carcinoma Caki-2 cells. The results suggest that chrysophanic acid induces necrosis through reactive oxygen species (ROS) generation, and the underlying cellular processes do not coincide with those of apoptosis or necroptosis.
MATERIALS AND METHODS
1. Reagents
Chrysophanic acid and anti-β-actin antibody were purchased from Sigma-Aldrich (St. Louis, MO, USA). Antibodies against murine double minute-2 (Mdm2), p53, p27, and horseradish peroxidase (HRP)-conjugated secondary antibodies were obtained from Santa Cruz Biotechnology (Paso Robles, CA, USA). Other antibodies were from Cell Signaling Technology (Beverly, MA, USA), and 2′,7′-dichlorofluorescein diacetate (DCF-DA) was from Invitrogen (Carlsbad, CA, USA). Hank’s balanced salt solution (HBSS) was from Meditech (Herndon, VA, USA).
2. Cell culture
Human renal clear cell carcinoma Caki-2 cells were purchased from American Type Culture Collection (Manassas, VA, USA) and maintained in Dulbecco’s modified Eagle’s medium supplemented with 10% FBS and a mixture of antibiotics (100 U/mL of penicillin G and 100 mg/mL of streptomycin) at 37°C under a humidified atmosphere containing 5% CO2. Caki-2 cells were plated at 1 × 105 cells/well on a 6-well plate and incubated with chrysophanic acid at concentrations specified below at 50% to 60% confluency in all experiments.
3. Cell viability assay
The cytotoxic effect of chrysophanic acid on Caki-2 cells was measured by MTT assay. Cells were plated at 2 × 103 cells/well onto a 96-well plate and incubated. After 24 hours of incubation, cells were incubated with the indicated concentration of chrysophanic acid at 37°C for 24 hours or 48 hours. Then, 10 μL of MTT stock solution (5 mg/mL) was added to each well, followed by further incubation for 4 hours. After removal of the media, water insoluble formazan was dissolved in dimethyl sulfoxide and absorbance at 550 nm was measured with a microplate reader (Tecan Trading AG, Männedorf, Switzerland). Cell viability was calculated as the percentage relative to control based on independent triplicate experiments.
4. Detection of cell death by Annexin V-propidium iodide staining
Annexin V-propidium iodide (PI) staining was used to assess the profile of cell death caused by chrysophanic acid. Chrysophanic acid-treated cells were harvested by trypsinization and washed three times with PBS. The cells were then resuspended in binding buffer supplied by the manufacturer and stained with fluorescein isothiocyanate (FITC)-conjugated anti-Annexin V antibody and PI from the FITC-Annexin V staining kit (BD Biosciences, San Jose, CA, USA) in accordance with the manufacturer’s instructions. Fluorescence intensity was measured with a FACSVerse (BD Biosciences).
5. Measurement of reactive oxygen species generation
Cells were treated with chrysophanic acid at the indicated concentration for 24 hours and harvested by trypsinization. After washing, the harvested cells were stained with 25 μM of DCF-DA for 30 minutes. After that, the cells were washed three times with HBSS and resuspended in fresh HBSS. The fluorescence of DCF-DA was measured at Ex/Em = 480 nm/525 nm with a FACSVerse (BD Biosciences).
6. Immunoblot analysis
Cells treated with chrysophanic acid were harvested and lysed with radioimmunoprecipitation assay buffer, and the lysates were clarified by centrifugation at 4°C, 12,000 rpm, for 20 minutes. The quantity of protein in lysates was determined by Bradford assay (Pierce Biotechnology, Rockford, IL, USA). A total of 30 μg of each lysate was mixed with SDS PAGE loading buffer and denatured at 100°C for 5 minutes. The lysates were separated by 12% or 15% SDS PAGE and transferred onto a polyvinylidene difluoride (PVDF) membrane. The PVDF membranes were blocked with 5% non-fat dry milk in 10 mM Tris and 150 mM NaCl (pH 7.5) with 0.1% Tween-20 (TBS-T) for 1 hour at room temperature. The membranes were then hybridized with each antibody (1:1,000) at 4°C for 12 hours. After hybridization, the membranes were washed thrice with TBS-T and subsequently hybridized with the corresponding HRP-conjugated secondary antibodies (1:5,000) at room temperature for 2 hours. The membranes were incubated with enhanced chemiluminescence (GE Healthcare, Little Chalfont, UK) and the results of immunoblotting were visualized with ImagequantTM LAS 4000 (Fujifilm Life Science, Tokyo, Japan).
7. Statistical analysis
Results are expressed as mean ± SD, and Student’s t-test was used for single comparisons. A probability value of P < 0.05 was used as the criterion for statistical significance.
RESULTS
1. Chrysophanic acid causes cell death in Caki-2 renal carcinoma cells
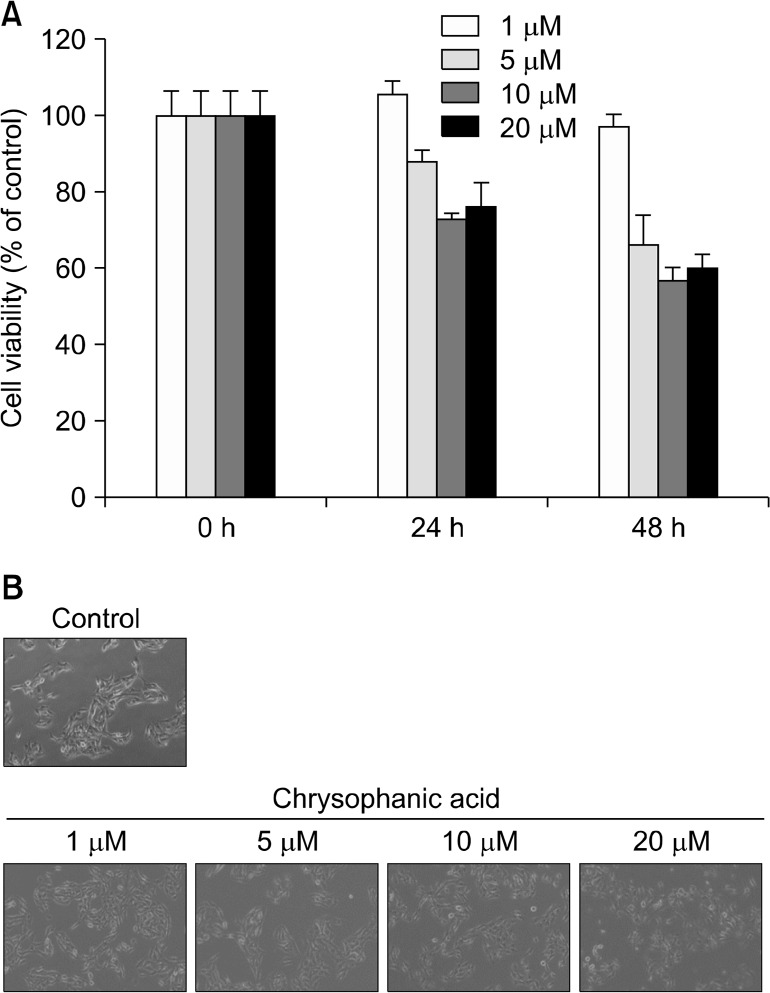
To confirm that chrysophanic acid induces cell death in Caki-2 cells, cell viability after treatment with chrysophanic acid was analyzed by MTT assay. Treatment of Caki-2 cells with chrysophanic acid showed a significant concentration- and time-dependent reduction in viability in comparison to untreated cells (Fig. 1A). Moreover, the morphological changes in Caki-2 cells that were treated with chrysophanic acid resembled the typical characteristics of dead cells. At a concentration 20 μM of chrysophanic acid, the viability of Caki-2 cells was reduced by approximately 40%.
Figure 1.
Chrysophanic acid induces cell death in Caki-2 cells. (A) Caki-2 cells were treated with the indicated concentrations of chrysophanic acid for 24 or 48 hours. Cell viability was determined by MTT assay. Values are expressed as means ± SD. (B) Cells were treated with the indicated concentrations of chrysophanic acid for 24 hours and cell morphology was assessed by phase contrast microscopy (× 200).
2. Chrysophanic acid induces propidium iodide-positive necrotic cell death
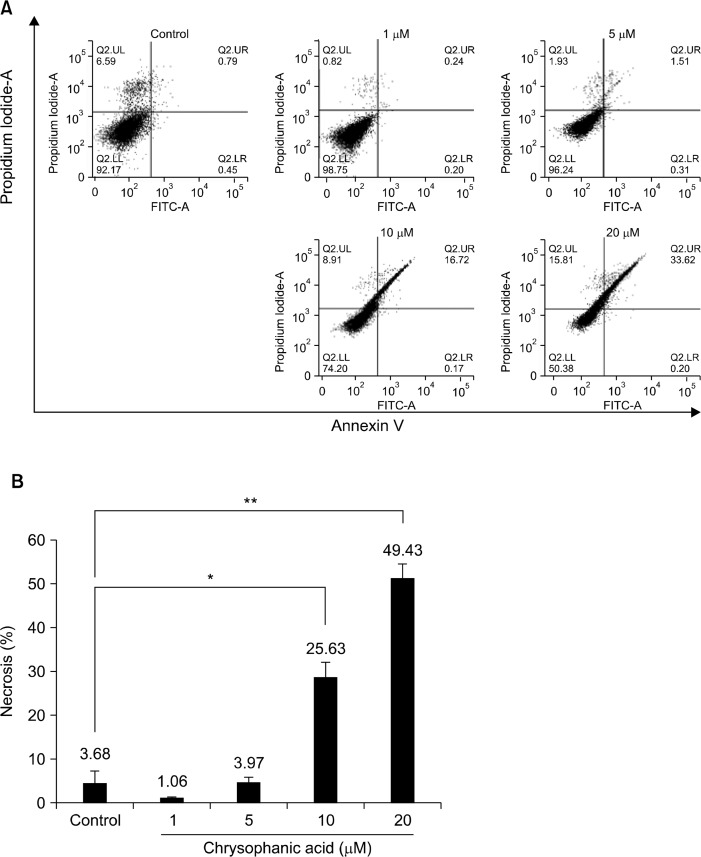
To characterize the cytotoxic effect of chrysophanic acid on Caki-2 cells, chrysophanic acid-treated cells were subjected to Annexin V-PI staining and fluorescence-activated cell sorting (FACS) analysis. Caki-2 cells were treated with 0, 1, 5, 10, or 20 μM of chrysophanic acid for 24 hours and stained with FITC-conjugated anti-Annexin V antibody and PI to calculate the proportion of cells undergoing necrosis. The results of FACS analysis showed that chrysophanic acid treatment dramatically increased PI-positive necrotic cell death in comparison to untreated control cells (Fig. 2A). The proportion of necrotic cells and the results of the statistical analysis are shown in Figure 2B.
Figure 2.
Chrysophanic acid-induced cell death exhibits features of necrotic cell death. (A) The cell death index (%) was analyzed by flow cytometry after treatment with chrysophanic acid (1, 5, 10, or 20 μM) for 24 hours. Cells were stained with fluorescein isothiocyanate (FITC) conjugated anti-Annexin V antibody and propidium iodide. (B) The cell death index (%) was plotted after statistical analysis. The data represent three independent experiments, and mean values are marked on the graph (*P < 0.05, **P < 0.001).
3. Reactive oxygen species contribute to chrysophanic acid-induced cell death in Caki-2 cells
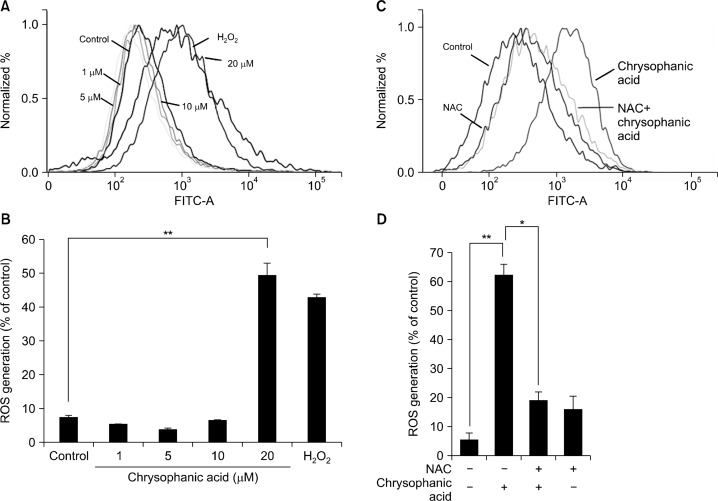
To assess the effect of chrysophanic acid on total ROS generation, Caki-2 cells were treated with chrysophanic acid and hydrogen peroxide was also applied as a control. Thereafter, the cells were stained with DCF-DA and subjected to FACS analysis. Treatment with 20 μM chrysophanic acid for 24 hours induced cells to generate high levels of ROS (Fig. 3A). In cells treated with 20 μM chrysophanic acid, ROS generation increased by approximately five-fold in comparison to control cells (Fig. 3B). To investigate whether pretreatment with N-acetyl cysteine (NAC) suppresses chrysophanic acid-induced ROS generation, cells were treated with NAC before application of chrysophanic acid. NAC pretreatment reduced chrysophanic acid-induced ROS generation by approximately 70% (Fig. 3C and 3D).
Figure 3.
Chrysophanic acid induces the generation of reactive oxygen species (ROS) in Caki-2 cells. (A) Cells were treated with chrysophanic acid (1, 5, 10, or 20 μM) or hydrogen peroxide for 24 hours, and the generation of intracellular ROS was analyzed by flow cytometry. (B) Changes in fluorescence intensity of dichlorofluorescin diacetate after chrysophanic acid treatment were calculated and plotted. (**P < 0.001). (C) Cells were treated with 5 mM N-acetyl cysteine (NAC) for 1 hour, then treated with 20 μM of chrysophanic acid. Intracellular ROS levels were measured by flow cytometry. (D) The graph shows the generation of intracellular ROS due to chrysophanic acid treatment in the presence or absence of NAC. The data are presented as mean ± SD (*P < 0.05, ** P < 0.001).
4. Chrysophanic acid activates neither apoptosis nor necroptosis in Caki-2 cells
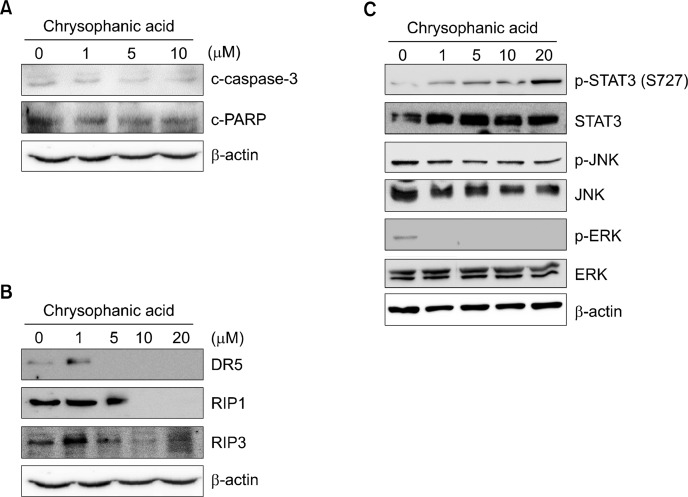
To confirm that chrysophanic acid induces necrotic cell death, the underlying cellular processes associated with different types of cell death were investigated. The proteolytic activation of caspase-3 (c-caspase-3), cleaved PARP (c-PARP), and the expression of p53 were examined as representative apoptotic markers. As shown in Fig. 4A, no changes in the levels of c-caspase-3 and c-PARP were detected following chrysophanic acid-induced cell death. p53 was not detected. Next, we assessed necroptotic marker proteins, in particular the presence of proteolytically cleaved caspase-8 (c-caspase-8) and the expression levels of DR5 and receptor-interacting serine/threonine-protein kinase 1 (RIP1) and 3 (RIP3). In this experiment, c-caspase-8 was not detected at any concentration of chrysophanic acid, and the expression levels of DR5, RIP1, and RIP3 were lower after treatment with chrysophanic acid (Fig. 4B). Finally, the phosphorylation and expression level of transcription factors or signal transducers that contribute to ROS-mediated cell death, in particular of STAT3, c-Jun N-terminal kinase (JNK), and extracellular signal-regulated kinase (ERK), were investigated. Our data showed that phosphorylation of serine residue 727 (S727) of STAT3 increased in a dose-dependent manner, whereas S727 phosphorylation of STAT3 is generally reduced during apoptosis. Moreover, the expression of STAT3 remained constant and served as a control at all concentrations of chrysophanic acid. The phosphorylated forms and expressions of JNK and ERK were unaffected by treatment of chrysophanic acid (Fig. 4C).
Figure 4.
Chrysophanic acid did not alter expression levels or post-translational modifications of proteins in apoptosis- and necroptosis-related pathways. (A) Cells were treated with the indicated concentrations of chrysophanic acid, and then the levels of representative markers of apoptosis, namely, cleaved PARP and caspase-3. were detected by immunoblotting. (B) The levels of proteins that are involved in the necroptosis pathway, namely, death receptor 5 (DR5), receptor-interacting serine/threonine-protein kinase 1 (RIP1), and RIP3, were analyzed by immunoblotting. (C) The expression levels and phosphorylation of ROS-related transcription factor, STAT3, and signaling mediators, c-Jun N-terminal kinase (JNK) and extracellular signal-regulated kinase (ERK), were investigated. Beta-actin was used as a loading control.
DISCUSSION
Cancer is one of the leading causes of death worldwide.22 Despite efforts to develop new treatments, cancer chemotherapy frequently induces a wide range of side effects or drug resistance. Many investigators therefore try to find new therapeutics and useful natural products. This study characterized the effect of chrysophanic acid, a component of the medicinal plants R. officinale or P. cuspidatum, on human RCC, Caki-2 cells and sought to uncover its mechanism of action. Previous studies have reported that chrysophanic acid induces apoptosis in rat hepatocytes and human promyeloleukemic HL-60 cells,23,24 and necrosis in human lung cancer A549, human liver cancer J5, and Hep3B cells.12,13,25 However, the underlying molecular and cellular processes remain poorly understood.
Apoptosis is categorized into two different pathways, namely, the extrinsic and the intrinsic pathway. The extrinsic pathway is triggered by the activation of DRs, while the intrinsic pathway is activated by DNA damage, ROS, and manifold stress.26 The intrinsic pathway is executed by sequential activation of the caspase cascade and accompanied by an alteration in mitochondrial membrane potentials and the release of cytochrome c from the mitochondria into the cytoplasm.27–29 Necrosis differs from apoptosis and is not associated with its typical characteristics, namely, activation of caspase-3, chromatin condensation, and membrane blebbing. In addition, necrosis may arise as a consequence of inflammation, infection, or physical damage.30 Necroptosis is a regulated form of necrosis, which has been observed in developmental processes and following tissue damage,31,32 and is regulated by RIP1, RIP3, and mixed lineage kinase domain-like protein.33,34
The present study has shown that chrysophanic acid exerts a cytotoxic effect on Caki-2 cells, and dead cells after treatment with chrysophanic acid were found to be PI-positive (Fig. 1 and 2). As necrotic cells are typically PI-positive,35 chrysophanic acid-induced cell death was deemed to be necrosis-like. Moreover, elevated intracellular ROS levels due to chrysophanic acid might contribute to necrosis-like cell death. ROS play a critical role in necrosis.36 General markers of apoptosis (Fig. 4A) and necroptosis (Fig. 4B) were assessed by immunoblot analysis to elucidate the mechanism of cell death associated with chrysophanic acid. However, significant alterations in these markers were not detected. Furthermore, quantification of STAT3, a well-known transcription factor involved in cell proliferation- or anti-apoptotic signaling pathways, and stress-induced signal transducing kinases JNK and ERK showed that chrysophanic acid-induced cell death did not resemble necroptosis nor apoptosis.37–40 The involvement of these molecular markers of apoptosis/necroptosis is ruled out from this study, and it appears chrysophanic acid has a cell-type specific effect.
In conclusion, the present study demonstrates that chrysophanic acid induces necrosis-like cell death in human RCC Caki-2 cells, and the necrosis due to chrysophanic acid appears not to share the signaling pathways of apoptosis or necroptosis.
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea Government (MSIP) (No. NRF-2014R1A1A1008685).
Footnotes
CONFLICTS OF INTEREST
No potential conflicts of interest were disclosed.
REFERENCES
- 1.Motzer RJ, Bander NH, Nanus DM. Renal-cell carcinoma. N Engl J Med. 1996;335:865–75. doi: 10.1056/NEJM199609193351207. [DOI] [PubMed] [Google Scholar]
- 2.Mulders P, Figlin R, deKernion JB, Wiltrout R, Linehan M, Parkinson D, et al. Renal cell carcinoma: recent progress and future directions. Cancer Res. 1997;57:5189–95. [PubMed] [Google Scholar]
- 3.Rini BI, Rathmell WK, Godley P. Renal cell carcinoma. Curr Opin Oncol. 2008;20:300–6. doi: 10.1097/CCO.0b013e3282f9782b. [DOI] [PubMed] [Google Scholar]
- 4.Lam JS, Leppert JT, Belldegrun AS, Figlin RA. Novel approaches in the therapy of metastatic renal cell carcinoma. World J Urol. 2005;23:202–12. doi: 10.1007/s00345-004-0466-0. [DOI] [PubMed] [Google Scholar]
- 5.Santoni M, De Tursi M, Felici A, Lo Re G, Ricotta R, Ruggeri EM, et al. Management of metastatic renal cell carcinoma patients with poor-risk features: current status and future perspectives. Expert Rev Anticancer Ther. 2013;13:697–709. doi: 10.1586/era.13.52. [DOI] [PubMed] [Google Scholar]
- 6.Huang Q, Lu G, Shen HM, Chung MC, Ong CN. Anti-cancer properties of anthraquinones from rhubarb. Med Res Rev. 2007;27:609–30. doi: 10.1002/med.20094. [DOI] [PubMed] [Google Scholar]
- 7.Tang T, Yin L, Yang J, Shan G. Emodin, an anthraquinone derivative from Rheum officinale Baill, enhances cutaneous wound healing in rats. Eur J Pharmacol. 2007;567:177–85. doi: 10.1016/j.ejphar.2007.02.033. [DOI] [PubMed] [Google Scholar]
- 8.Lee MS, Cha EY, Sul JY, Song IS, Kim JY. Chrysophanic acid blocks proliferation of colon cancer cells by inhibiting EGFR/mTOR pathway. Phytother Res. 2011;25:833–7. doi: 10.1002/ptr.3323. [DOI] [PubMed] [Google Scholar]
- 9.Shoemaker M, Hamilton B, Dairkee SH, Cohen I, Campbell MJ. In vitro anticancer activity of twelve Chinese medicinal herbs. Phytother Res. 2005;19:649–51. doi: 10.1002/ptr.1702. [DOI] [PubMed] [Google Scholar]
- 10.Fosse C, Le Texier L, Roy S, Delaforge M, Grégoire S, Neuwels M, et al. Parameters and mechanistic studies on the oxidative ring cleavage of synthetic heterocyclic naphthoquinones by Streptomyces strains. Appl Microbiol Biotechnol. 2004;65:446–56. doi: 10.1007/s00253-004-1588-4. [DOI] [PubMed] [Google Scholar]
- 11.Arosio B, Gagliano N, Fusaro LM, Parmeggiani L, Tagliabue J, Galetti P, et al. Aloe-Emodin quinone pretreatment reduces acute liver injury induced by carbon tetrachloride. Pharmacol Toxicol. 2000;87:229–33. doi: 10.1034/j.1600-0773.2000.d01-79.x. [DOI] [PubMed] [Google Scholar]
- 12.Lu C, Yang JS, Huang AC, Hsia TC, Chou ST, Kuo CL, et al. Chrysophanol induces necrosis through the production of ROS and alteration of ATP levels in J5 human liver cancer cells. Mol Nutr Food Res. 2010;54:967–76. doi: 10.1002/mnfr.200900265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ni CH, Chen PY, Lu HF, Yang JS, Huang HY, Wu SH, et al. Chrysophanol-induced necrotic-like cell death through an impaired mitochondrial ATP synthesis in Hep3B human liver cancer cells. Arch Pharm Res. 2012;35:887–95. doi: 10.1007/s12272-012-0514-z. [DOI] [PubMed] [Google Scholar]
- 14.Nikoletopoulou V, Markaki M, Palikaras K, Tavernarakis N. Crosstalk between apoptosis, necrosis and autophagy. Biochim Biophys Acta. 2013;1833:3448–59. doi: 10.1016/j.bbamcr.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 15.Earnshaw WC, Martins LM, Kaufmann SH. Mammalian caspases: structure, activation, substrates, and functions during apoptosis. Annu Rev Biochem. 1999;68:383–424. doi: 10.1146/annurev.biochem.68.1.383. [DOI] [PubMed] [Google Scholar]
- 16.Leist M, Jäättelä M. Four deaths and a funeral: from caspases to alternative mechanisms. Nat Rev Mol Cell Biol. 2001;2:589–98. doi: 10.1038/35085008. [DOI] [PubMed] [Google Scholar]
- 17.Los M, Mozoluk M, Ferrari D, Stepczynska A, Stroh C, Renz A, et al. Activation and caspase-mediated inhibition of PARP: a molecular switch between fibroblast necrosis and apoptosis in death receptor signaling. Mol Biol Cell. 2002;13:978–88. doi: 10.1091/mbc.01-05-0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–57. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wyllie AH, Kerr JF, Currie AR. Cell death: the significance of apoptosis. Int Rev Cytol. 1980;68:251–306. doi: 10.1016/S0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]
- 20.Laster SM, Wood JG, Gooding LR. Tumor necrosis factor can induce both apoptic and necrotic forms of cell lysis. J Immunol. 1988;141:2629–34. [PubMed] [Google Scholar]
- 21.Jouan-Lanhouet S, Arshad MI, Piquet-Pellorce C, Martin-Chouly C, Le Moigne-Muller G, Van Herreweghe F, et al. TRAIL induces necroptosis involving RIPK1/RIPK3-dependent PARP-1 activation. Cell Death Differ. 2012;19:2003–14. doi: 10.1038/cdd.2012.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 23.Kågedal K, Bironaite D, Ollinger K. Anthraquinone cytotoxicity and apoptosis in primary cultures of rat hepatocytes. Free Radic Res. 1999;31:419–28. doi: 10.1080/10715769900300981. [DOI] [PubMed] [Google Scholar]
- 24.Chen YC, Shen SC, Lee WR, Hsu FL, Lin HY, Ko CH, et al. Emodin induces apoptosis in human promyeloleukemic HL-60 cells accompanied by activation of caspase 3 cascade but independent of reactive oxygen species production. Biochem Pharmacol. 2002;64:1713–24. doi: 10.1016/S0006-2952(02)01386-2. [DOI] [PubMed] [Google Scholar]
- 25.Ni CH, Yu CS, Lu HF, Yang JS, Huang HY, Chen PY, et al. Chrysophanol-induced cell death (necrosis) in human lung cancer A549 cells is mediated through increasing reactive oxygen species and decreasing the level of mitochondrial membrane potential. Environ Toxicol. 2014;29:740–9. doi: 10.1002/tox.21801. [DOI] [PubMed] [Google Scholar]
- 26.Ouyang L, Shi Z, Zhao S, Wang FT, Zhou TT, Liu B, et al. Programmed cell death pathways in cancer: a review of apoptosis, autophagy and programmed necrosis. Cell Prolif. 2012;45:487–98. doi: 10.1111/j.1365-2184.2012.00845.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ghobrial IM, Witzig TE, Adjei AA. Targeting apoptosis pathways in cancer therapy. CA Cancer J Clin. 2005;55:178–94. doi: 10.3322/canjclin.55.3.178. [DOI] [PubMed] [Google Scholar]
- 28.Wang X. The expanding role of mitochondria in apoptosis. Genes Dev. 2001;15:2922–33. [PubMed] [Google Scholar]
- 29.Garrido C, Galluzzi L, Brunet M, Puig PE, Didelot C, Kroemer G. Mechanisms of cytochrome c release from mitochondria. Cell Death Differ. 2006;13:1423–33. doi: 10.1038/sj.cdd.4401950. [DOI] [PubMed] [Google Scholar]
- 30.Okada H, Mak TW. Pathways of apoptotic and non-apoptotic death in tumour cells. Nat Rev Cancer. 2004;4:592–603. doi: 10.1038/nrc1412. [DOI] [PubMed] [Google Scholar]
- 31.Zhou W, Yuan J. Necroptosis in health and diseases. Semin Cell Dev Biol. 2014;35:14–23. doi: 10.1016/j.semcdb.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 32.Sun L, Wang X. A new kind of cell suicide: mechanisms and functions of programmed necrosis. Trends Biochem Sci. 2014;39:587–93. doi: 10.1016/j.tibs.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 33.Sun L, Wang H, Wang Z, He S, Chen S, Liao D, et al. Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell. 2012;148:213–27. doi: 10.1016/j.cell.2011.11.031. [DOI] [PubMed] [Google Scholar]
- 34.Zhao J, Jitkaew S, Cai Z, Choksi S, Li Q, Luo J, et al. Mixed lineage kinase domain-like is a key receptor interacting protein 3 downstream component of TNF-induced necrosis. Proc Natl Acad Sci U S A. 2012;109:5322–7. doi: 10.1073/pnas.1200012109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vitale M, Zamai L, Mazzotti G, Cataldi A, Falcieri E. Differential kinetics of propidium iodide uptake in apoptotic and necrotic thymocytes. Histochemistry. 1993;100:223–9. doi: 10.1007/BF00269095. [DOI] [PubMed] [Google Scholar]
- 36.Vanlangenakker N, Vanden Berghe T, Krysko DV, Festjens N, Vandenabeele P. Molecular mechanisms and pathophysiology of necrotic cell death. Curr Mol Med. 2008;8:207–20. doi: 10.2174/156652408784221306. [DOI] [PubMed] [Google Scholar]
- 37.Kusaba T, Nakayama T, Yamazumi K, Yakata Y, Yoshizaki A, Inoue K, et al. Activation of STAT3 is a marker of poor prognosis in human colorectal cancer. Oncol Rep. 2006;15:1445–51. [PubMed] [Google Scholar]
- 38.Lin L, Liu A, Peng Z, Lin HJ, Li PK, Li C, et al. STAT3 is necessary for proliferation and survival in colon cancer-initiating cells. Cancer Res. 2011;71:7226–37. doi: 10.1158/0008-5472.CAN-10-4660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vlahopoulos S, Zoumpourlis VC. JNK: a key modulator of intracellular signaling. Biochemistry (Mosc) 2004;69:844–54. doi: 10.1023/B:BIRY.0000040215.02460.45. [DOI] [PubMed] [Google Scholar]
- 40.Sebolt-Leopold JS. Advances in the development of cancer therapeutics directed against the RAS-mitogen-activated protein kinase pathway. Clin Cancer Res. 2008;14:3651–6. doi: 10.1158/1078-0432.CCR-08-0333. [DOI] [PubMed] [Google Scholar]