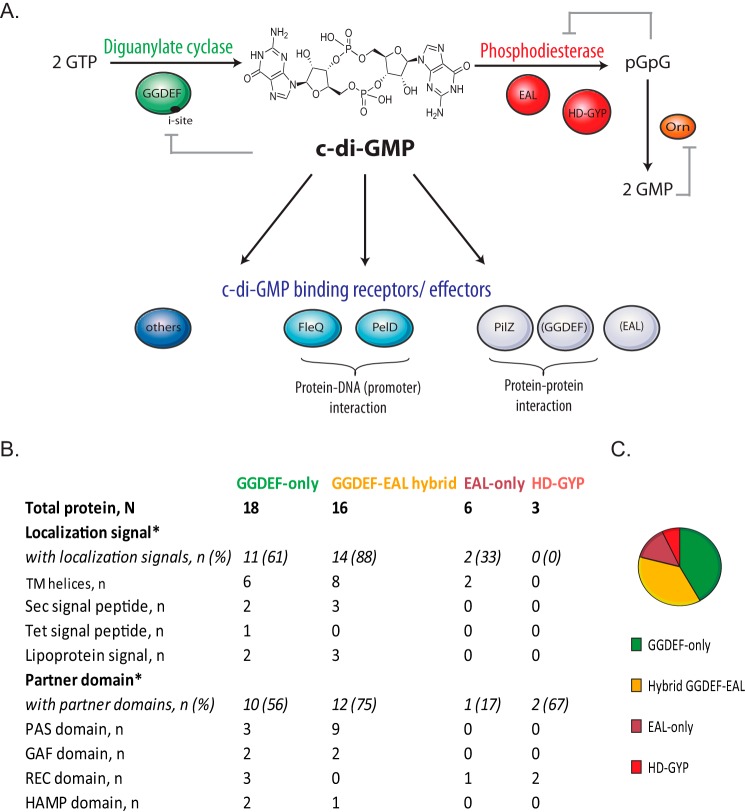
FIGURE 1.
Molecular basis of c-di-GMP signaling in P. aeruginosa. A, c-di-GMP is synthesized by diguanylate cyclases (green) that carry GGDEF domains and degraded by phosphodiesterases (red) that carry either EAL or HD-GYP domains. EAL phosphodiesterases linearize c-di-GMP into pGpG, which is successively hydrolyzed into 2 GMP molecules primarily by the oligoribonuclease Orn (orange) (34, 35). HD-GYP-phosphodiesterases are proposed to perform both steps of the c-di-GMP degradation process (31). Feedback inhibition mechanisms are illustrated by gray lines. In the cell, c-di-GMP regulates cellular processes at different levels (transcriptional, post-transcriptional, and post-translational). The diversity of c-di-GMP-binding receptors and effectors (blue) is the key of the c-di-GMP pleiotropic mechanisms. B, spatial localization signals and partner domain occurrence for GGDEF, EAL, and HD-GYP proteins of P. aeruginosa. Table based on the work of Seshasayee et al. (17) *: The sets of proteins corresponding to each of the category are not mutually exclusive. Organization of classes is in agreement as described previously (17). TM helices, transmembrane helices. C, pie chart illustrating numerical proportion of GGDEF, EAL, and HD-GYP proteins in P. aeruginosa.