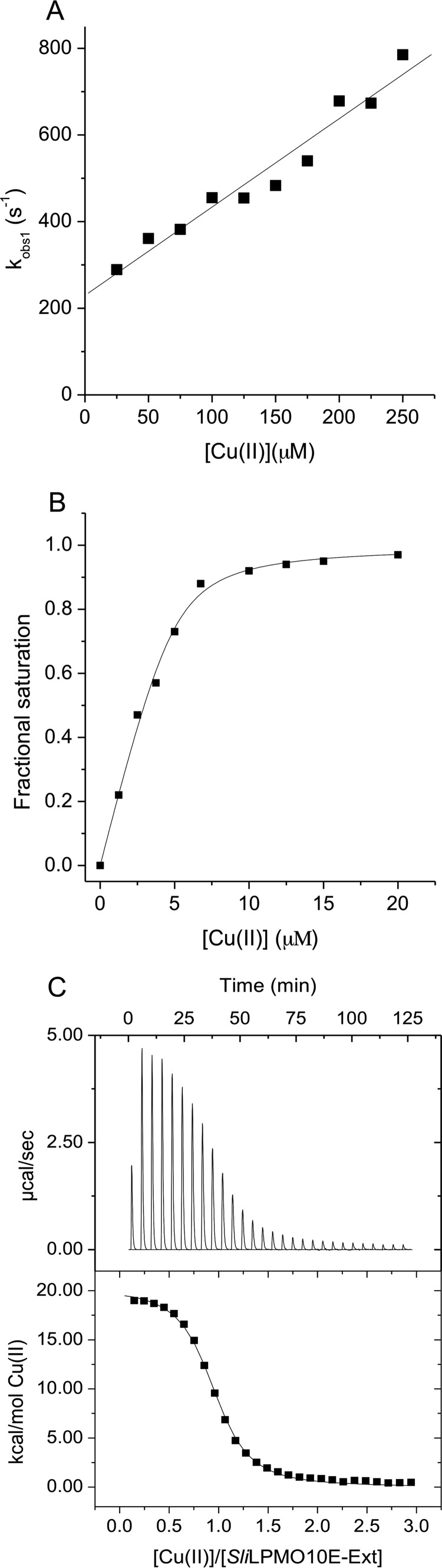
FIGURE 8.
Kinetics and thermodynamics of Cu(II) binding to SliLPMO10E-Ext at pH 5 and 25 °C. A, first-order rate constants for the fast (kobs, 1) phase of Cu(II) binding to SliLPMO10E-Ext as a function of [Cu(II)]. B, titration of Cu(II) to SliLPMO10E-Ext plotted as a function of fractional saturation defined as Y = 1 − ΔF/ΔFT, where ΔF is the fluorescence change and ΔFT is the fluorescent change from zero to fully saturated SliLPMO10E with Cu(II). C, ITC binding profile and fit to a single-site binding model (solid line) upon titrating SliLPMO10E-Ext with Cu(II). Thermodynamic parameters obtained from the fit are reported in Table 5.