FIGURE 10.
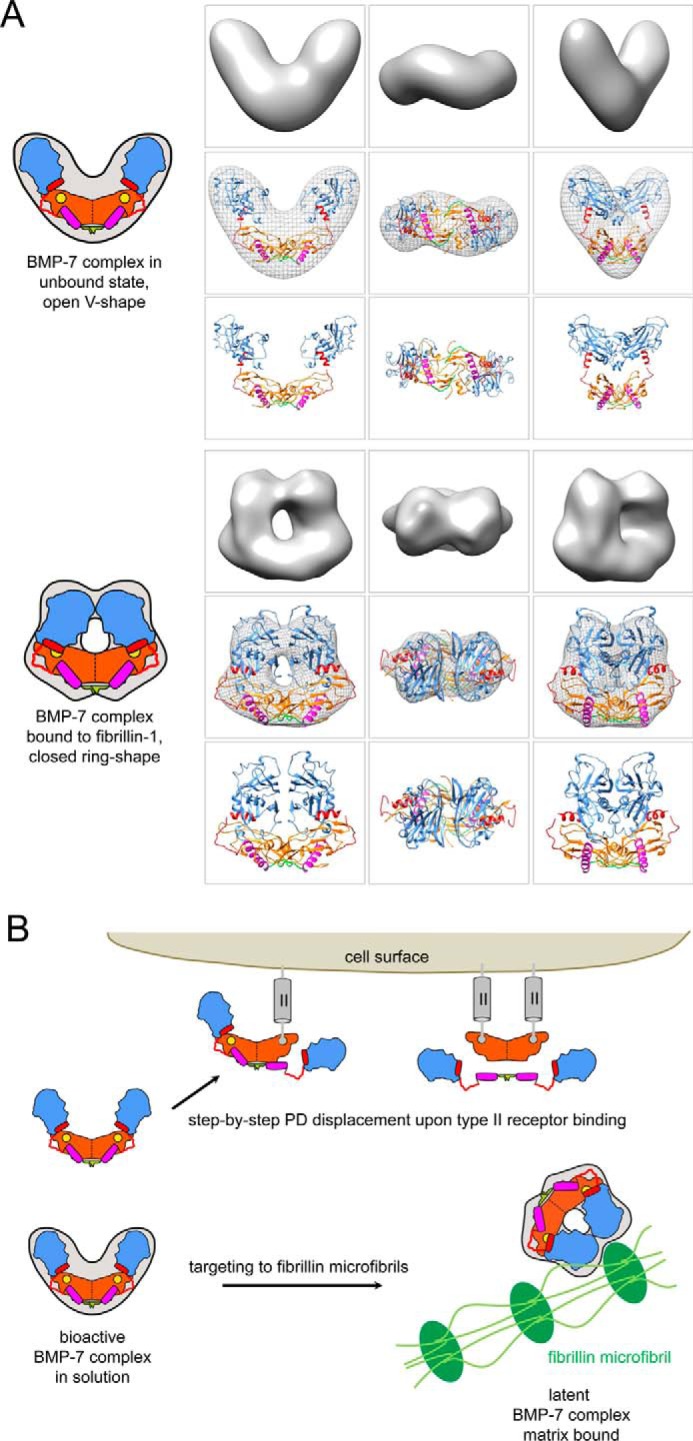
Homology models of the BMP-7 complex and model of extracellular control of BMP GF activity via PD interactions with fibrillin-1 microfibrils. A (top), in the unbound, bioactive state, the BMP-7 complex adopts an open V-like shape. In this conformation, the PDs are in contact with each other via the first 18 N-terminal residues (green). The GF shows an extended, open conformation similar to the TGF-β-1 GF in the SLC (34), which enables positioning of the α1-helix (purple rod) of the PD within a pocket of the GF. The PD contains a 65PHRP68 motif (red hinge) located between the α1- and α2-helix (red rod), which serves as an important “molecular clamp” for maintaining interaction with the GF and is therefore required for proper PD competition with type II receptor binding. In this conformation, the α2-helix is not occupying the type II receptor binding site on the GF. Bottom, upon binding to fibrillin-1, the BMP-7 complex undergoes a conformational change. In this latent, closed conformation, the two PD arms may interact with each other via unmasked C-terminal self-interaction epitopes, which in turn facilitate the ring closure. In the closed ring shape conformation, the α2 occupies the type II receptor binding site, which confers latency to the GF. B, in solution, binding of type II receptors to the GF moiety of the BMP-7 complex results in displacement of the PDs as a dimer. The PDs remain tethered to each other via their N-terminal self-interaction epitopes (green). Binding to fibrillin-1 microfibrils (green) induces a conformational change within the PD that enables a closed ring-shaped conformation of the BMP-7 complex, rendering the GF latent. Homology models of the BMP-7 complex in its open and closed forms were generated using the structure of the TGF-β-1 SLC (34) and fitted into the shapes determined by TEM. For the open BMP-7 form, the model is fitted in the electron density map from EM, and for the closed form the model is shown as electron density rendered at 20 Å resolution. Orange, GF dimer; yellow circle, type II receptor binding site; green, N-terminal self-interaction epitope; magenta, α1-helix; red, α2-helix; red, stretch connecting α1- and α2-helix containing the 65PHRP68 motif; light blue, C-terminal portion of BMP-7 PD.
