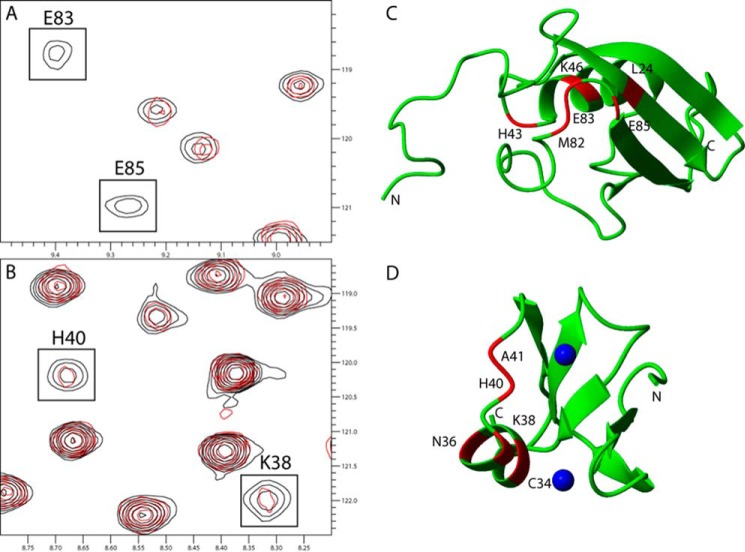
FIGURE 1.
Chemical shift differences observed when titrating the unlabeled ZZ domain or SUMO1 into 13C,15N-labeled SUMO1 or ZZ domain, respectively. A, cutout of 15N HSQC showing backbone amides in SUMO1 affected by the binding of the ZZ domain. B, cutout of 15N HSQC showing backbone amides in the ZZ domain affected by the binding of SUMO1. Contours colored in black correspond to the apo form, whereas contours colored in red correspond to ZZ domain-bound SUMO1. C, residues in SUMO1 affected by the binding of ZZ are highlighted in red: Leu-24, His-43, Lys-46, Met-82, Glu-83, and Glu-85 (Protein Data Bank code 1A5R). D, residues in the ZZ domain affected by the binding of SUMO1 are highlighted in red: Cys-34, Asn-36, Lys-38, His-40, and Ala-41. Zinc ions are depicted as blue spheres (Protein Data Bank code 1TOT). Protein images were made using Molmol (37).