FIGURE 7.
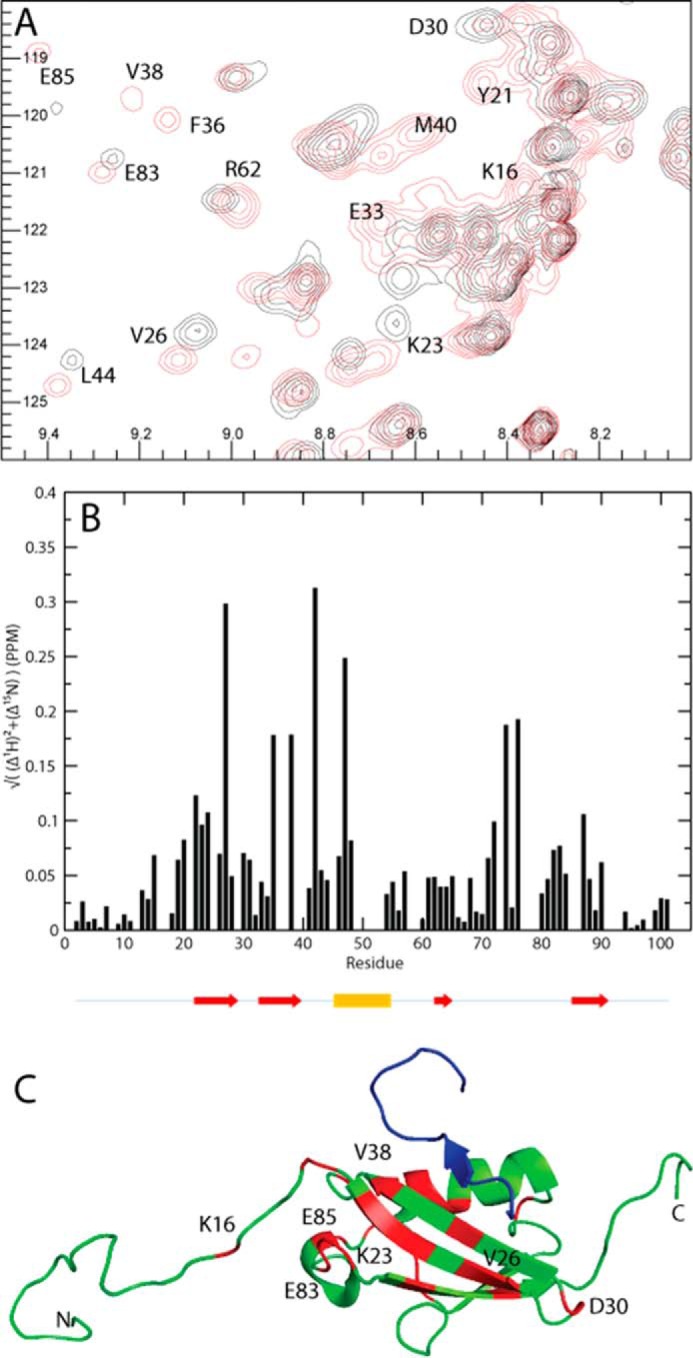
Chemical shift differences observed when titrating SIMPX into 13C,15N SUMO1. A, cutout of 15N HSQC of SUMO1 showing backbone amides in SUMO1 affected by the binding of SIMPX. Black corresponds to apo, and red corresponds to the SIMPX-bound SUMO1. The affected residues are indicated by their respective residue number. B, weighted 1H,15N chemical shift differences between the SIMPX and the apo state of SUMO1 plotted per backbone residue. The location for the secondary structure elements of SUMO1 are indicated by arrows (red) for β-strands and a cylinder for the single α-helix (yellow). C, SUMO1 is colored in green, residues with a significant weighted chemical shift differences (>0.05 PPM) are colored in red, where a subset is indicated by their respective residue number, whereas SIMPX is shown in blue (Protein Data Bank code 2ASQ).
