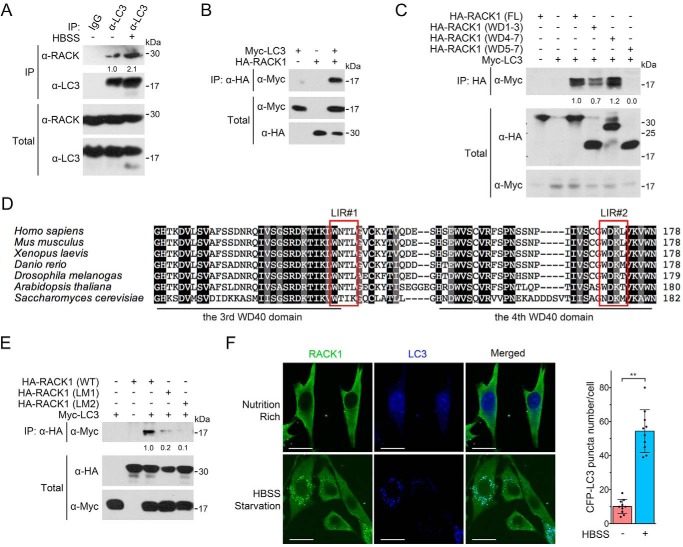
FIGURE 5.
RACK1 associates with LC3. A, HEK293T cells were starved in HBSS before being harvested for anti-LC3 immunoprecipitation followed by immunoblotting. BFA1 was added to HBSS to block LC3 degradation in starvation-induced autophagy. The total cell lysates were analyzed by IB. The protein levels of precipitated RACK1 were quantified and normalized against precipitated LC3, and the values are shown below the bands. B, HEK293T cells were transfected with Myc-LC3 and HA-RACK1 for 24 h before being harvested for IP followed by IB. The total cell lysates were also analyzed by IB. C, HEK293T cells were transfected with Myc-LC3 and HA-tagged wild-type or truncated RACK1 mutants. After 24 h, cells were harvested for IP-IB analysis. The total cell lysates were also analyzed by IB. The protein levels of precipitated Myc-LC3 were quantified and normalized to the corresponding total Myc-LC3 fragments, and the values are shown below the bands. FL, full-length. D, the amino acid sequences of RACK1 of human (P63244), mouse (P68040), frog (Q5U275), zebrafish (O42248), fruit fly (B4NZ46), Arabidopsis (O24456 and Q9LV28), and budding yeast (P38011) were aligned by ClustalX. The conserved LIR motif is boxed. E, the WXXL sequence of LIRs in human RACK1 was mutated to AXXA to generate HA-RACK1(LM1) and RACK1(LM2) mutants. HEK293T cells were transfected with Myc-LC3 and HA-RACK1/HA-RACK1(LM1/2). After 24 h, cells were harvested for IP and IB analyses. The protein levels of precipitated Myc-LC3 were quantified and normalized to total Myc-LC3, and the values are shown below the bands. F, NRK cells stably expressing CFP-LC3 were starved in HBSS for 2 h and then subjected to anti-RACK1 immunofluorescence. The CFP-LC3 puncta in each cell (n = 10 cells from three different slides) were counted, and the number of puncta per cell is represented as the mean ± S.D. in the right panel. Two-tailed unpaired Student's t test was used in statistical analyses (**, p < 0.01). Scale bars = 10 μm.