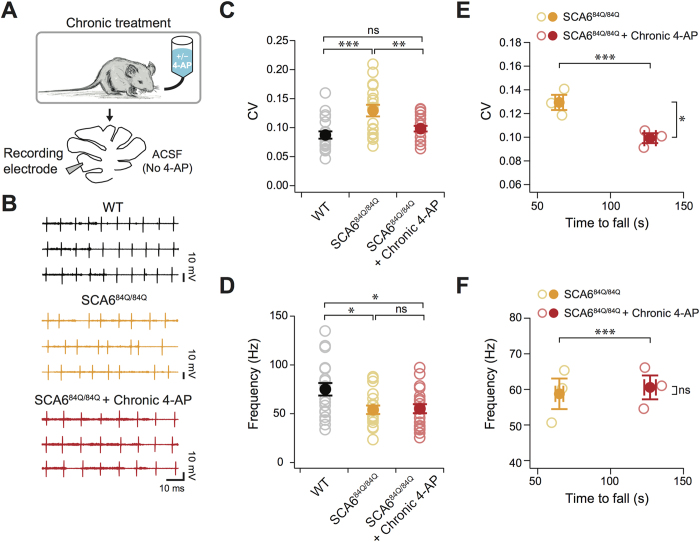
Figure 5. Chronic 4-AP restores in vitro Purkinje cell firing precision in SCA684Q/84Q mice.
(A) After chronic 4-AP or vehicle treatment for 3 months, Purkinje cell action potentials were recorded in the absence of 4-AP in the extracellular solution in acute slices. (B) Sample recordings from age-matched WT (top, black traces), vehicle-treated SCA684Q/84Q (middle, orange traces), and chronic 4-AP-treated SCA684Q/84Q mice (bottom, red traces) showing that the firing precision is recovered in 4-AP-treated mice. (C) Spike precision, as measured by the CV of inter-spike intervals from Purkinje cell spike trains from WT, vehicle-treated SCA684Q/84Q, and 4-AP-treated SCA684Q/84Q mice, shows a significant reduction after 4-AP treatment to levels that are indistinguishable from WT (WT: CV = 0.09 ± 0.01; SCA684Q/84Q with vehicle: CV = 0.13 ± 0.01; SCA684Q/84Q + 4-AP: CV = 0.10 ± 0.01). (D) Firing frequency is however unaffected by 4-AP treatment in SCA684Q/84Q mice, and both show significant reduction from WT levels (WT: frequency = 76.6 ± 5.5 Hz; SCA684Q/84Q with vehicle: frequency = 58.8 ± 3.7 Hz; SCA684Q/84Q + 4-AP: frequency = 59.7 ± 3.9 Hz). N = 20 for WT, N = 18 for untreated SCA684Q/84Q, and N = 20 for SCA684Q/84Q + 4-AP for (C,D). (E,F) For individual animals, 4-AP treatment produced a significant increase in the time spent on the Rotarod (X-axis for both graphs, P < 0.0005). (E) For individual mice, 4-AP treatment significantly reduced the average CV of recorded Purkinje cells (red) from untreated (orange) SCA684Q/84Q mice (P = 0.011 for CV), while (F), there was no significant difference on an animal-by-animal basis for Purkinje cell firing frequency (P = 0.75 for frequency). For (E,F), open circles show data for individual mice, while filled circle show averages. N = 5–8 Purkinje cells/animal for N = 3 vehicle-treated and N = 3 4-AP-treated SCA684Q/84Q mice; Comparisons made with one-way ANOVA followed by post-hoc Tukey test for (C,D), and Student’s t test for (E,F); *P < 0.05; **P < 0.01; ***P < 0.005.