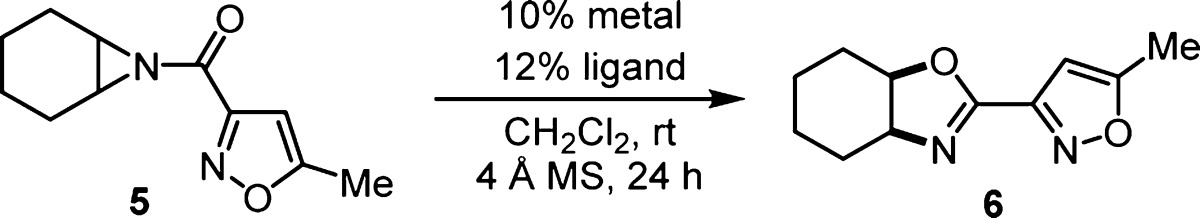Table 1. Catalyst Discovery for an Enantioselective Heine Reactiona.
| entry | metal | ligand | conversion (%)b | ee (%)b |
|---|---|---|---|---|
| 1 | Cu(OTf)2 | L1 | 12 | 28 |
| 2 | La(OTf)3 | L1 | 24 | –33 |
| 3 | Cu(OTf)2 | L2 | 95 | 39 |
| 4 | Cu(OTf)2 | L3 | 93 | 82 |
| 5 | Ni(OTf)2 | L3 | 23 | –35 |
| 6 | Pd(OTf)2 | L3 | 41 | 60 |
| 7 | Pd(OTf)2 | L4 | 29 | 85 |
| 8 | Pd(OTf)2 | L5 | 27 | 95 |
| 9 | 7 | — | 0 | — |
| 10 | Pd(OTf)2 | L6 | 33 | 92 |
| 11c | Pd(OTf)2 | L6 | 79 | 94 |
| 12c | Pd(ClO4)2 | L6 | 60 | 76 |
| 13 | Pd(BF4)2 | L6 | 0 | — |
| 14c,d | Pd(OTf)2 | L6 | 89 | 88 |
Reaction conditions: aziridine 5 (0.1 mmol) and 50 mg of 4 Å powdered molecular sieves were added to the preformed catalyst (0.01 mmol of metal and 0.012 mmol of ligand) in CH2Cl2 (0.2 M) at room temperature for 24 h.
Determined by HPLC peak area ratios of 5 and 6 at 250 nm using the CHIRALCEL OJ column.
Reaction solvent was toluene.
Reaction performed with 5 mol % catalyst.