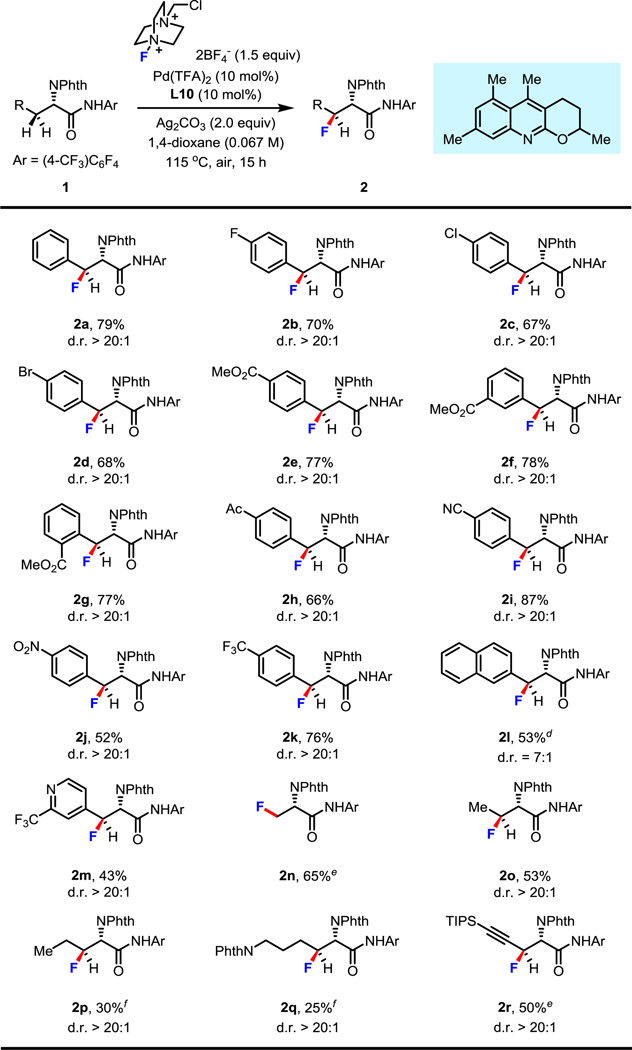
Table 2.
Condition A: 0.1 mmol of 1, 1.5 equiv of Selectfluor, 10 mol% of Pd(TFA)2, 10 mol% of L10, 2.0 equiv of Ag2CO3, 1.5 mL of 1,4-dioxane, 115 °C, under air, 15 h.
Isolated yields.
The d.r. value was estimated by 1H NMR analysis of the crude product.
Isolated yield of major diastereomer.
Condition B: 0.1 mmol of 1, 1.25 equiv of Selectfluor, 10 mol% of Pd(OAc)2, 20 mol% of L5, 1.25 mL of 1,4-dioxane, 115 °C, under air, 15 h.
Condition A, but using 20 mol% of L10 and adding 1.0 equiv of KHCO3.