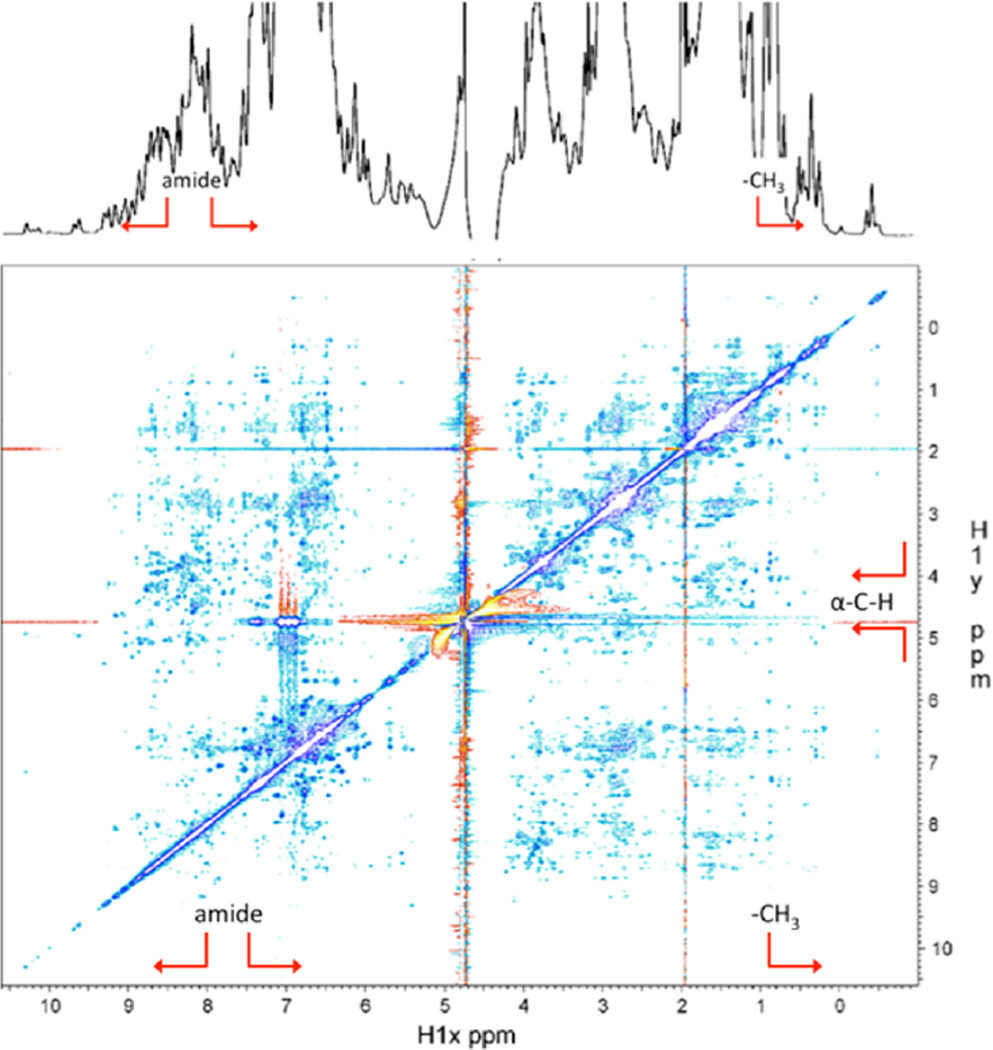
Figure 5.
1D and 2D NOESY NMR spectra of Mfp-6 at pH 3. The chemical shifts are dispersed over a wide range and show an extensive network of NOEs for the amide (above 8–8.5 ppm, red arrow brackets) and methyl protons (below ~1 ppm, red arrow), indicative of a well-folded domain. The downfield shifts of the Cα protons above ~5 ppm (red arrow brackets) further point to the presence of extended β-strand structure. The 1D spectrum was recorded on a Varian Inova 600 MHz spectrometer. The 2D NOESY spectrum was recorded with a mixing time of 150 ms on a Bruker 800 MHz spectrometer. The protein concentration was 0.5 mM in 85% H2O, 10% D2O, and 5% d4-acetic acid.