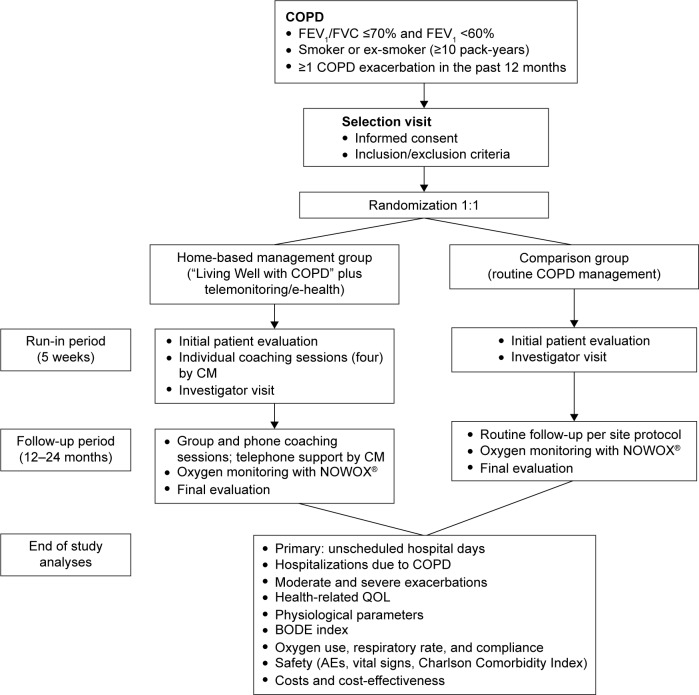
Figure 1.
Design of the COMET study.
Notes: COPD patients are randomized 1:1 into a home-based management group and a comparison group. Following patient education, the home-based management group provides proactive health status updates weekly or in cases of symptom worsening. Patients reporting worsening or alarm status receive a telephone call by a CM and confirmed cases of alarm status are referred to the investigator for same-day medical assessment and management. Outcomes will be analyzed after all patients have completed their follow-up period of 2 years (maximum) and the study is complete.
Abbreviations: CM, case manager; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; QOL, quality of life; BODE, body mass index, airflow obstruction, dyspnea, and exercise capacity; AE, adverse events; COMET, COPD patient Management European Trial.