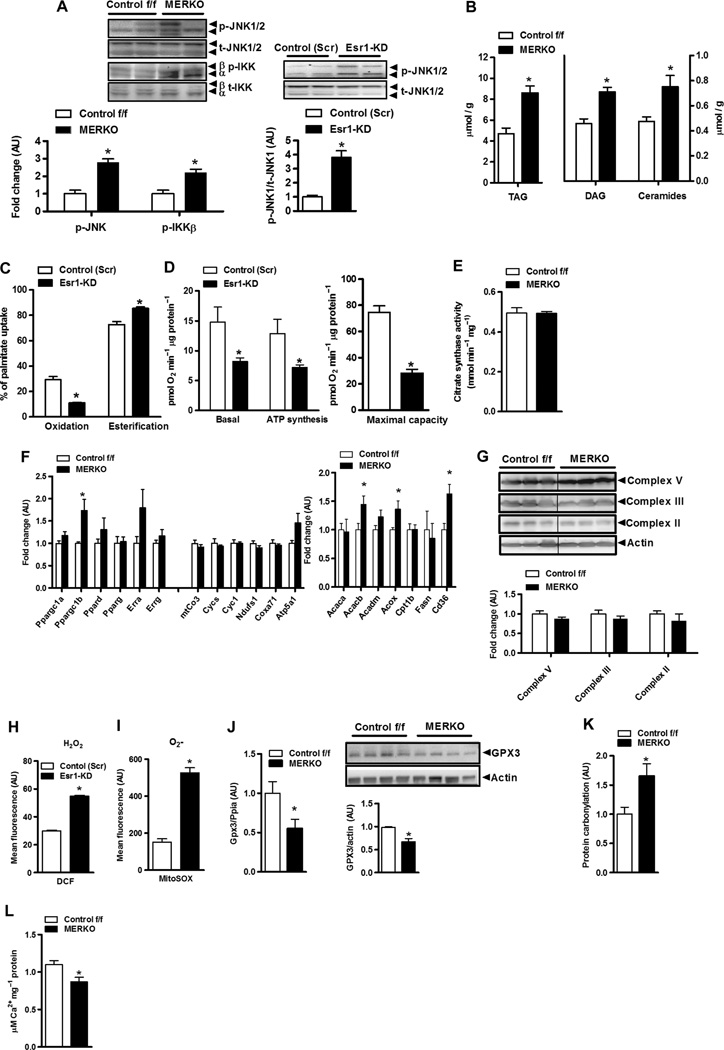
Fig. 3. Esr1 deletion promotes mitochondrial dysfunction, ROS production, and increased muscle inflammation.
(A) Heightened proinflammatory signaling (p-IKKβ and p-JNK 1/2) (fold change over baseline normalized to 1.0) in female MERKO muscle (n = 6 per genotype; left panel) and myotubes (n = 4 per condition; right panel), respectively. P < 0.05. (B) Bioactive lipid intermediates (TAG, triacylglycerol; ceramides) in quadriceps muscle from female Control f/f and MERKO mice (n = 6 muscles pergenotype). P < 0.05. (C and D) Palmitate oxidation and esterification as well as basal and maximal (stimulated using carbonylcyanide p-trifluoromethoxyphenylhydrazone, FCCP) oxygen consumption and ATP synthesis in control (Scr) versus Esr1-KDmyotubes (n = 6). (E) Citrate synthase activity in muscle harvested from Control f/f versus MERKO mice (n = 6 per genotype). (F) Quantitative reverse transcription PCR (RT-PCR) analyses of muscle from Control f/f versus MERKO mice (n = 6 per genotype). (G) Immunoblots and corresponding densitometry of mitochondrial electron transport complexes versus actin loading control in muscle from Control f/f versus MERKO mice (n = 6 per genotype). (H and I) H2O2 and O2− production in control (Scr) versus Esr1- KD C2C12 myocytes. (J) Gpx3 expression and protein in Control f/f versus MERKO muscle (n = 6 per genotype). (K) Oxidative stress–induced protein carbonylation in quadriceps muscle from Control f/f versus MERKO mice (n = 8 per genotype). (L) Calcium buffering capacity/mPTP opening in mitochondria isolated from Control f/f versus MERKO mice (n = 5 per genotype). All values are expressed as means ± SEM. Mean differences detected by Student’s t test and ANOVA where appropriate; *P < 0.05.