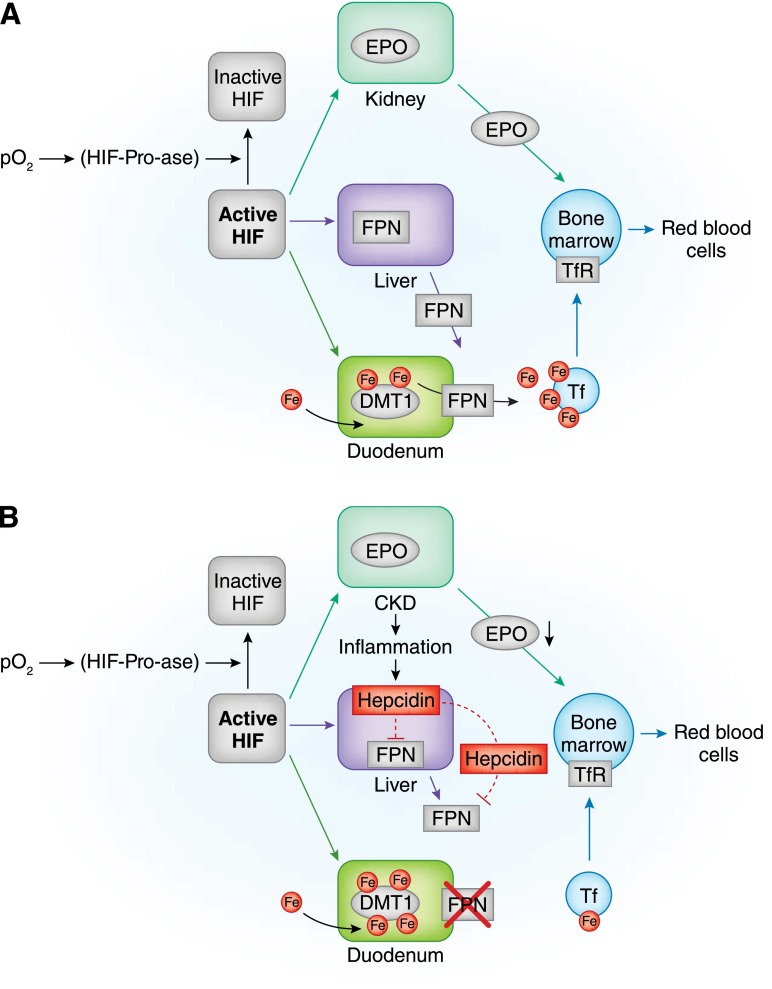
Figure 1.
Anemia, erythropoiesis, and iron absorption in normal subjects and patients with CKD. (A) In normal subjects, by inhibiting hypoxia–inducible factor–prolyl–hydroxylases (HIF-Pro-ase), hypoxia stabilizes hypoxia-inducible factor (HIF), thus increasing its activity. In time, the HIF signaling cascade increases renal synthesis of erythropoietin (EPO), liver synthesis of ferroportin (FPN), and duodenal synthesis of divalent metal transporter 1 (DMT1). By targeting duodenal cells, FPN favors iron transfer into blood. In blood, iron is bound to transferrin (Tf) and transported to transferrin receptor (TfR) –expressing cells. Bone marrow cells supplied with iron and stimulated by EPO can efficiently increase red blood cell production. (B) In chronic inflammatory states, like chronic renal failure , increments in HIF do not efficiently increase renal synthesis of EPO or liver synthesis of FPN. This last effect also results from increased liver synthesis of epcidin, an inflammatory cytokine capable of inhibiting both FPN liver synthesis and its binding to duodenal cells. Intestinal absorption of iron is blunted with possibly increased intracellular iron concentrations. With lower iron availability and inadequate EPO stimulation, bone marrow cannot efficiently increase red blood cell production. (C) Iron supply in chronic renal failure can be obtained by either oral or intravenous administration. The oral route can be regarded as physiologic but is hampered by reduced efficiency of intestinal absorption and gastrointestinal side effects; the unphysiologic intravenous administration is hindered by possible toxic effects through production of labile plasma iron (LPI). Lines indicate activation, and dashed lines indicate inhibition. Fe, iron; I.V., intravenous; pO2, partial pressure of oxygen.