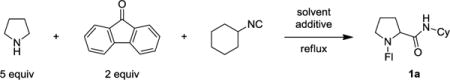
Table 1.
Evaluation of Reaction Conditionsa
| ||||
|---|---|---|---|---|
| entry | solvent (molarity) |
additive (equiv) |
time [h] |
yield 1a (%) |
| 1 | PhMe (0.1) | – | 36 | trace |
| 2 | PhMe (0.1) | AcOH (0.2) | 48 | 6 |
| 3 | PhMe (0.1) | AcOH (1) | 48 | 28 |
| 4 | PhMe (0.1) | AcOH (5) | 20 | 73 |
| 5 | PhMe (0.1) | AcOH (10) | 20 | 52 |
| 6 | xylenes (0.1) | AcOH (5) | 20 | 53 |
| 7 | n-BuOH (0.1) | AcOH (5) | 20 | 55 |
| 8b | DMF (0.1) | AcOH (5) | 18 | 52 |
| 9 | PhMe (0.1) | 2-EHA (5) | 20 | 32 |
| 10 | PhMe (0.1) | BzOH (5) | 20 | 63 |
| 11 | PhMe (0.25) | AcOH (5) | 18 | 89 |
| 12 | PhMe (0.5) | AcOH (5) | 15 | 75 |
| 13c | PhMe (0.25) | AcOH (5) | 20 | 53 |
| 14d | PhMe (0.25) | AcOH (5) | 20 | 85 |
Reactions were performed with 0.5 mmol of cyclohexylisocyanide. Yields are isolated yields of chromatographically purified compounds.
Reaction was performed at 135 °C.
With 3 equiv of pyrrolidine.
With 10 equiv of H2O.
