Table 1.
Influence of Nucleophile and Ligand on Reactivitya
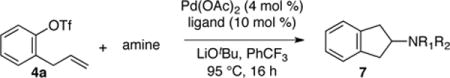
| |||||
|---|---|---|---|---|---|
|
| |||||
| entry | amine | ligand | T (°C) | conv (%)a,b | yield (%)a |
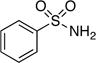
| 1 |
|
RuPhos | 95 | 0 | – |
| 2 |
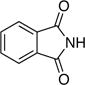
|
CPhos | 95 | 0 | – |
| 3 |
|
RuPhos | 95 | 0 | – |
| 4 |
|
RuPhos | 95 | 80 | 45 |
| 5 |
|
CPhos | 95 | 85 | 62 |
| 6 |
|
JackiePhos | 95 | 100 | 45 |
| 7 |
|
BrettPhos | 95 | 100 | 73 (68) |
| 8 |
|
BrettPhos | 40 | 32 | 5 |
| 9 |
|
BrettPhos | 70 | 65 | 70 |
| 10c |
|
BrettPhos | 95 | 100 | quant. |
Conditions: 1.0 equiv 4a, 1.0 equiv amine, 1.4 equiv LiOtBu, 4 mol % Pd(OAc)2, 10 mol % ligand, PhCF3 (0.1 M), 95 °C, 16 h.
Determined by 1H NMR using phenanthrene as an internal standard. Isolated yields are given in parentheses.
1.0 equiv of 2-allylphenyl triflate and 1.2 equiv of pyrrolidine were used.
