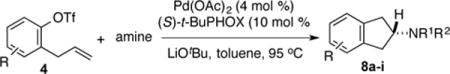
Table 3.
Asymmetric Synthesis of Aminoindanesa
| ||||
|---|---|---|---|---|
|
| ||||
| entry | substrate | amine | % yieldb | er |
| 1 2c |
|
|
98 95 |
>99:1 99:1 |
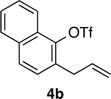
| 3 | 4b |
|
88 | 95:5 |
| 4 | 4b |
|
90 | 95:5 |
| 5 | 4b |
|
47 | 89:11 |
| 6 | 4b |
|
63 | >99:1 |
| 7 | 4b |
|
82 | 97:3 |
| 8 |
|
|
56 | 97:3 |
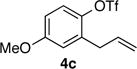
| 9 | 4c |
|
59 | >99:1 |
| 10 |
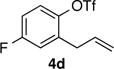
|
|
77 | 98:2 |
Conditions: Reactions were conducted on a 0.1 mmol scale using 1.0 equiv 4, 1.2 equiv amine, 1.4 equiv LiOtBu, 4 mol % Pd(OAc)2, 10 mol % (S)-tBu-PHOX, toluene (0.1 M), 95 °C, 3–16 h.
Isolated yields (average of two or more experiments).
The reaction was conducted on a 1.0 mmol scale.
