Fig 2. Latency is established with higher efficiency following co-culture of resting CD4+ T-cells with mDC.
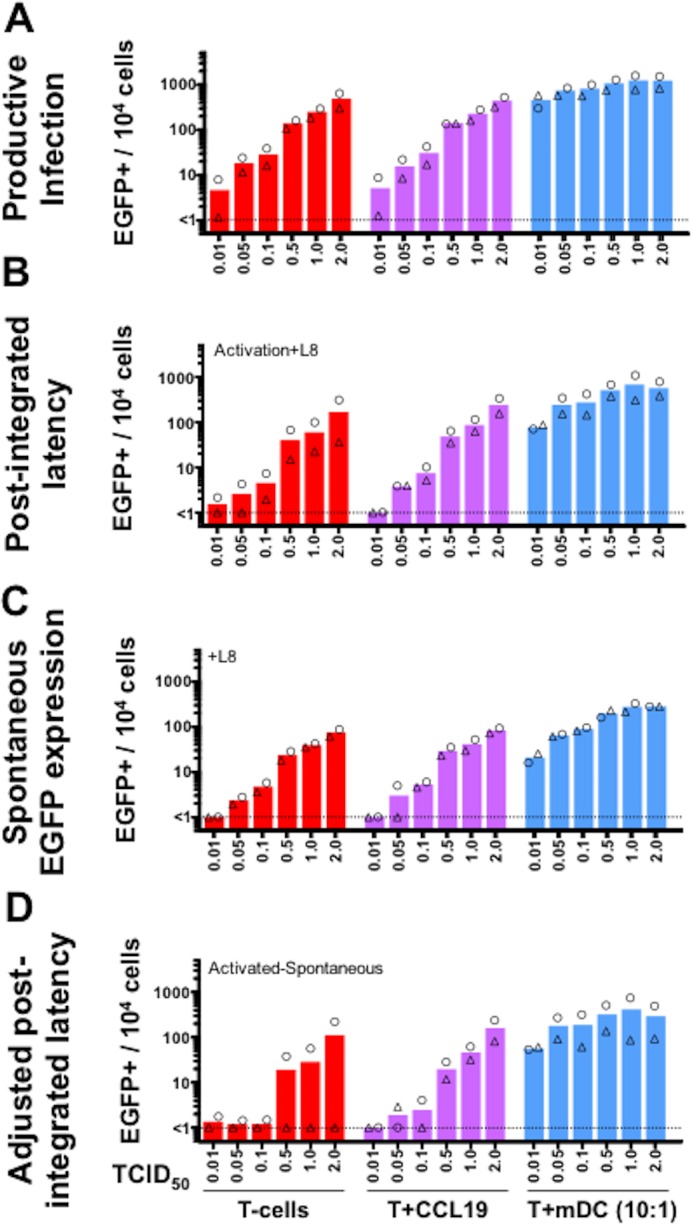
Resting CD4+ T-cells from 2 donors were labelled with eFluor670 cytoplasmic dye and cultured for 24 hours either untreated (red), with 100 nM CCL19 (purple) or with autologous mDCs at 1 mDC:10 T-cell ratio (blue). Cells were incubated with increasing TCID50 per cell of X4-tropic HIVNL4.3-EGFP. After 2 hours, cells were washed, cultured for 5 days in a low concentration of IL-2 (2 U/ml) and analysed for EGFP expression by flow cytometry to quantify productive infection (A). Additionally, non-proliferating eFluor670hiEGFP- cells were sorted, cultured for 3 days with anti-CD3/anti-CD28 plus IL-7, in the presence of the integrase inhibitor L-870812 (L8), to induce EGFP expression from post-integration latent infection (B). As a comparative control, aliquots of sorted cells were also cultured for 3 days with L-870812 (L8) but no reactivation stimuli in order to measure background, spontaneous EGFP expression during 3 further days of culture (C). The true level of post-integrated latency in cultures was calculated by subtracting the percentage of EGFP+ cells in the spontaneous cultures from the percentage of EGFP+ cells in reactivated cultures (D). The percentage of EGFP+ cells per 104 cultured cells is shown. Columns represent the median of donor pairs with each donor shown as a different symbol.
