Abstract
Cytomegalovirus is the most common congenital infection causing serious disease in infants. It is the leading infectious cause of sensorineural hearing loss and neurodevelopmental disability in developed countries. Despite the clinical importance of congenital cytomegalovirus, surveys show there is limited awareness and knowledge in the medical and general community about congenital cytomegalovirus infection. This article reviews the clinical features, global epidemiology, transmission and risk factors for cytomegalovirus infections. It also highlights several major advances made in recent years in the diagnosis and prevention of cytomegalovirus infection during pregnancy. Although research is ongoing, no therapy is currently proven to prevent or treat maternal, fetal or neonatal cytomegalovirus infection. Education of women regarding hygiene measures can help prevent cytomegalovirus infection and are currently the best strategy to prevent congenital cytomegalovirus disease.
Keywords: Infection, infectious diseases, maternal–fetal medicine, neonatal medicine
Human CMV infection
Human CMV is a virus that infects most of the human population at some stage in their lives. It is a member of the Herpesviridae family of viruses, which includes herpes simplex virus type 1 and type 2, Varicella Zoster Virus, Epstein–Barr virus, Roseolovirus (HHV-6 and HHV-7), and Kaposi’s sarcoma-associated herpesvirus or HHV-8.1,2 Initial infection (also known as primary infection) consists of a period of active virus growth with virus shedding in saliva, breast milk, urine, genital secretions, and presence in blood (the viraemic phase), most of which are asymptomatic.3 CMV secretion from saliva, cervix, stool and urine can be constant or intermittent and last for weeks in adults but may continue for months or years in young children.4
CMV is not cleared from the host, but persists throughout life in a resting (latent) form. That is, any person who has CMV antibody present in serum (seropositive) has had previous infection and has latent virus present somewhere in their body. Latent infection is characterized by either a low level or absence of detectable virus growth in peripheral blood mononuclear cells (CD14+) and cells in the bone marrow (CD34+ and CD33+).5 Latent CMV may become reactivated and grow after stimuli such as inflammation or immune impairment due to pregnancy, some diseases, medical treatment with immunomodulating agents such as corticosteroids, chemotherapy and immunosuppressive therapy post-transplantation.6 Hormonal changes related to pregnancy may also stimulate reactivation of CMV, since viral secretion in urine and cervical–vaginal fluids increases during pregnancy with increasing gestational age.7 Sequence variability across the large viral genome generates extensive viral strain diversity,8,9 which may allow re-infection of the infected person by another strain of CMV (non-primary infection).6
Infection with CMV is generally asymptomatic in immunocompetent people, although clinical symptoms of primary infection can include a glandular fever (mononucleosis) syndrome characterized by flu-like symptoms, or occasionally persistent fever. Laboratory tests may show elevated lymphocyte counts (lymphocytosis) and/or elevated liver transaminase levels.10 Despite the generally clinically silent nature in immunocompetent people, CMV infection is a significant health issue in immunocompromised individuals, where there is increased risk of morbidity and mortality.6 In addition, CMV infection has been associated with long-term consequences including immune senescence, age-related alteration and dysfunction of the immune system, leading to reduced protective immunity, however this is still under investigation.11,12
Key issues of cytomegalovirus infection during pregnancy
Knowledge and awareness of cytomegalovirus (CMV) infection during pregnancy is limited among parents.
CMV is present in latent form in all seropositive mothers.
CMV is the leading infectious cause of congenital malformation.
Transmission to the fetus occurs more often upon primary CMV infection than virus reactivation or re-infection with a different strain.
Hygienic precautions such as hand washing and wearing gloves after changing nappies reduce incidence of primary CMV infection of seronegative women.
Epidemiology of CMV infections
CMV is a global infection, with seropositivity in women of reproductive age ranging from 45 to >90%.1,13 CMV seroprevalence varies between countries and tends to be higher in developing countries (>90% in Brazil, 70–80% in Ghana, >90% in India, 80–90% in South Africa and >90% in Turkey) and lower in developed countries (40–70% in Western Europe, 60–70% in Australia, 60–70% in Canada and 50–60% in the United States).13 Even within countries the rates of CMV seropositivity in women vary by socio-economic status and ethnicity.13,14 Seroconversion, representing a primary (first) infection, occurs annually in approximately 1–2% of seronegative pregnant women.15
Anytime during pregnancy, primary or non-primary maternal infection (i.e. reactivation of a woman’s latent virus or re-infection with a different strain) can lead to CMV crossing the placental barrier and infecting the fetus, resulting in congenital CMV (cCMV) infection.16–18 The prevalence of cCMV has been reported to occur in 0.2–2% (average of 0.64%) of pregnancies in the US, Canada, Australia and Western Europe.19–22 In addition, the limited studies in developing countries, including Latin America (Chile, Brazil, Mexico and Panama), Africa (Ivory Coast and Gambia) and Asia (Korea, Taiwan, China and India) have reported a birth prevalence of cCMV infection ranging from 0.6 to 6.1% of pregnancies.23 Based on the number of live births per year24 and reported cCMV prevalence,20–23 this translates to an estimated ∼0.12 million cCMV infections annually in developed regions and ∼0.7–4.5 million cCMV infections per year in those developing countries that report their infection rates.
Congenital CMV transmission and disease
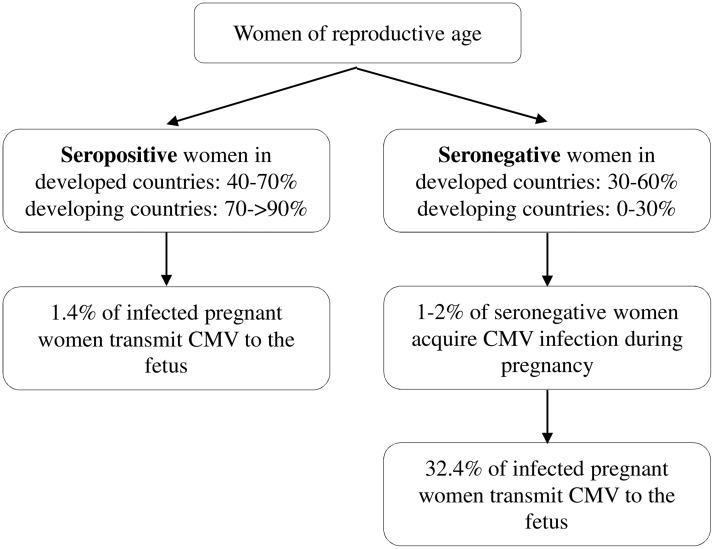
The risk of CMV in utero transmission leading to severe adverse fetal and neonatal outcome is reported to be much greater for primary maternal infection during the first half of pregnancy compared to non-primary infections or infection in the second half of pregnancy.25 Transmission to the fetus occurs in 14.2–52.4% (average of 32.4%) of primary infections, whereas 1.1–1.7% (average of 1.4%) of non-primary infections lead to fetal infection (Figure 1).20 However, considering the high seroprevalence of CMV, it is estimated that more than two-thirds of CMV-infected newborns are born to mothers who are already CMV seropositive. Therefore, non-primary infections are likely to contribute to more cases of cCMV disease.17,26
Figure 1.
Relationship of maternal seroprevalence and the risk of intrauterine cytomegalovirus transmission during pregnancy. Transmission to the fetus occurs in 14.2–52.4% (average 32.4%) of women who seroconvert during pregnancy, whereas transmission occurs in 1.1–1.7% (average 1.4%) of pregnant women who have a recurrent infection (reactivation of latent virus or re-infection with a different CMV strain).13,20
Congenital CMV infection may result in fetal death in utero, neonatal death, intrauterine growth restriction, preterm birth and maternal pregnancy complications including preeclampsia.27–32 Approximately 10% of infected newborns are symptomatic, with findings including unilateral or bilateral sensorineural hearing loss, vision loss, optic atrophy, strabismus, chorioretinitis, microcephaly, hepatomegaly, splenomegaly, thrombocytopenia, petechiae, jaundice, seizures and mental disability (Table 1).19,20,32 Additionally, approximately 15% of initially asymptomatic CMV-infected newborns develop long-term neurological sequelae before the age of 5 years.13,19,21,33 Clinical injury to the developing fetus may result from damage caused by the virus directly to the CMV-infected fetus.34 However, increasing evidence indicates that fetal injury can result from indirect effects through placental infection and placental dysfunction.27,35–37 CMV is now the leading non-genetic cause of congenital malformation in developed countries.13 Despite this, the extent of the problem with cCMV infection is largely unrecognized by mothers, fathers, obstetricians, midwives, paediatricians, medical students and other clinicians.1,20,38–40
Table 1.
| • Adverse pregnancy outcomes including stillbirth, neonatal death, intrauterine growth restriction and preterm birth. |
| • Maternal pregnancy complications such as preeclampsia. |
| • Fetal injury including: |
| • SNHL |
| • Vision loss, optic atrophy, strabismus and chorioretinitis |
| • Hepatomegaly and splenomegaly |
| • Thrombocytopenia |
| • Petechiae and jaundice |
| • Microcephaly, seizures and mental disability. |
SNHL: sensoneural hearing loss.
Transmission and risk factors for maternal CMV infection
CMV can be transmitted via body fluids from a person who is shedding CMV through breast feeding, close contact, sexual activity, blood transfusion and organ transplantation.6 Recent risk estimates for maternal primary infection demonstrated seronegative mothers with children in day care are at significant risk of becoming infected with CMV. Children attending day care frequently acquire CMV from other children.4 Approximately 50% of mothers with a CMV-infected child less than 2 years of age attending day care seroconvert within 1 year of commencement at day care.41 In addition to contact with urine or saliva of a child who is secreting CMV, a CMV-seropositive partner is an additional possible risk factor for seroconversion during pregnancy, as CMV is present in semen, and can be transmitted sexually.15
Preconception testing and maternal screening for CMV
Preconception screening for CMV can assist in pre-pregnancy counselling and guide in preventive measures for seronegative women. A recent cohort study, conducted at a fertility clinic, reported that advising recently seroconverted women (1.4%) to postpone fertility treatment prevented exposure to primary infection in early pregnancy. In addition, none of the seronegative women in this cohort (15.5%), who were educated on hygiene measures to reduce exposure to CMV, seroconverted during the 1 year follow-up.42
Additionally, identification of maternal primary infection during pregnancy assists in determining the risk of in utero transmission to the developing fetus and the possibility of fetal abnormalities. Our advancing knowledge of cCMV now fulfils many of the World Health Organization criteria outlined in papers by Wilson and Jungner43 commonly applied to universal screening test – CMV is an important health problem, diagnostic testing is available and the natural history of cCMV is known. However, preconception testing and universal screening of pregnant women for CMV is not recommended due to the absence of treatment for intrauterine CMV infection and remains a controversial issue.44 Discussion about who and how to screen continues in Australia and elsewhere.44,45 Nevertheless, in parts of Europe and Israel pregnant women are routinely serologically screened for CMV, with the objective of prenatal diagnosis.46,47
Diagnosis of maternal CMV infection
Serological tests that detect CMV-specific immunoglobulin G (IgG) antibody in initially seronegative women, can reliably confirm primary CMV infection, although such matched tests over time are rarely available.48 In order to ascertain baseline IgG seronegativity, a serum sample prior to conception or from the earliest antenatal visit is required, which is unavailable for most pregnant women. In the absence of a baseline sample, the detection of reactive CMV-specific IgM antibody may indicate a recent maternal CMV infection. However, reactive CMV IgM antibodies may be detected upon both primary and non-primary infections, and may persist for a long period of time (years) in some women following primary infection.49 Therefore, the presence of reactive CMV IgM antibodies should be complemented by determining the maturity (avidity) of CMV IgG antibodies.50 Increasingly tests for ‘IgG avidity’ are used to distinguish primary infection from reactivation in IgM seropositive women. These tests use the fact that in the first 18–20 weeks following infection CMV IgG antibodies that bind less tightly with their target protein are produced (low-affinity CMV IgG antibodies). Subsequently, CMV IgG antibodies with higher avidity (>60% binding in most assays) are generated. Therefore, IgM positivity and low-affinity CMV IgG antibodies indicate a recent primary infection, and when detected before 12–16 weeks of gestation indicate a high risk for congenital infection.51,52
Prenatal detection of fetal CMV infection
When a primary maternal infection is diagnosed or suspected, accurate detection of a CMV-infected fetus is important for further evaluation and informative parental counselling on expected outcomes of the pregnancy.53 It is recommended that referral is arranged to an expert with experience in the diagnosis and management of CMV infection in pregnancy, to ensure appropriate investigation and management is arranged. Numerous fetal structural and growth abnormalities have been described associated with cCMV infection, including intrauterine growth restriction, microcephaly, ventriculomegaly, periventricular calcification, echogenic bowel, polyhydramnios, hydrops, pleural effusion and placental enlargement.54,55 These abnormalities may be detected on a fetal ultrasound, however, these ultrasound features are non-specific.55,56 Ultrasound is an insensitive method for detecting cCMV, missing up to half of infected fetuses.55 In addition, fetal ultrasound abnormalities may only be detected late in the pregnancy and may change or even disappear with time.54,56 Moreover, lack of ultrasound findings does not exclude cCMV disease, since ∼15% of asymptomatic CMV-infected newborns later develop long-term neurological sequelae.13,19,21
The diagnosis of fetal CMV infection is best made using amniocentesis as CMV is excreted into the amniotic fluid through fetal urine. Therefore, amniocentesis should be performed after 20–21 weeks of gestation, once fetal urination is well established. Transmission of CMV from mother to the fetus may not occur immediately upon maternal infection, since CMV is not present at detectable levels in the amniotic fluid until 6–9 weeks after infection of the mother. Thus, to avoid false negative results, amniocentesis to diagnose cCMV infection should be performed at least 6 weeks after primary maternal infection and after approximately 21 weeks of gestation.57 Amniotic fluid is usually tested for the presence of CMV using nucleic acid tests (NAT) such as Polymerase Chain Reaction (PCR), but can also be detected using older techniques such as virus culture or direct fluorescence. The efficacy of these methods has been evaluated in recent studies where virus cultures of amniotic fluid detected cCMV with a 77% sensitivity and specificity of 100%,58 whilst PCR detected cCMV in amniotic fluid with a sensitivity ranging between 75 and 100%, and a specificity between 67 and 100%, depending upon the PCR method used (nested, one-round or real-time PCR).59–61 False positive results for both these methods are rare and are mostly explained by contamination of amniotic fluid with maternal blood. For maximal accuracy, amniotic fluid should be tested for CMV using viral cultures and PCR, although in practice most laboratories only perform NAT (PCR) tests.62
Postnatal diagnosis of fetal CMV infection
Postnatal detection of cCMV infection may allow for early intervention to improve clinical outcomes for the infected newborn.45 The standard method for the diagnosis of cCMV infection in newborns is NAT such as PCR on newborn urine and blood, although virus culture of urine is the traditional reference test. CMV can be cultured from body fluids, including urine, saliva, cerebrospinal fluid, bronchoalveolar lavage fluid or tissue from biopsy specimens. As an adjunct to culture techniques, PCR amplification of CMV DNA from saliva or dried blood spots is a rapid method to diagnose cCMV infection, although the assay is insensitive, missing up to half of all infected newborns.63–65 Detection of CMV-specific IgM in neonatal serum may also disclose congenital infection. However, care must be taken when measuring antibody titres for the diagnosis of cCMV, since IgM antibodies are only present in 20–70% of infected newborns.66 Notably, detection of CMV in a newborn older than 2–3 weeks could be the result of intrapartum or breast milk acquired infection.67 Consequently, virological and serological tests will no longer distinguish congenital from postnatal CMV infection. Therefore, diagnostic testing should occur within the first 3–4 weeks of life, although earlier (<2 weeks age) testing is best if available.68
Prevention and treatment of intrauterine CMV infection
Vaccines are currently under development and antiviral treatment for pregnant women (using valaciclovir) is being evaluated in a randomized clinical trial (Clinical Trial Identifier: NCT01651585).69–71 Additionally, recent studies have focused on passive immunization of pregnant women with CMV infection with hyperimmune globulin (CMV HIG) to reduce the rate of vertical CMV transmission and improve the outcome of the newborn.72 CMV HIG is an expensive pooled, high-titre immunoglobulin preparation derived from donors with high levels of CMV antibody. A prospective non-randomized observational study reported that intravenous CMV HIG infusions to pregnant women with primary CMV infection reduced the rate of mother-to-fetus transmission to 16% from 40% in women who did not receive HIG, and that the risk of cCMV disease was decreased (from 50 to 3%).73 Subsequent non-randomized studies reported a reduction in the number of congenitally infected newborns born to mothers who had been treated with CMV HIG or improved outcomes in CMV-infected infants.74–77 On the contrary, the Congenital HCMV Infection Prevention (CHIP) study, a randomized controlled trial, did not demonstrate a significant reduction in the rate of transmission of CMV infection among women receiving CMV HIG compared to women receiving placebo.78 A meta-analysis of the only two controlled studies suggested some benefit of CMV HIG in primary infection, although the benefit detected was small.72 The CHIP study also reported a higher rate of preterm deliveries among the pregnant women who received CMV HIG.78 However, a recent retrospective observational study did not find an association between receipt of maternal CMV HIG and prematurity or growth restriction.79 An additional two randomized clinical trial studies of the prevention of cCMV infection are currently ongoing in Europe80 and the United States (ClinicalTrials.gov number, NCT01376778). The findings from these studies should further our understanding of the efficacy and safety of CMV HIG administration to prevent cCMV infection.
Prevention of maternal CMV infection
In the absence of proven therapeutic options, prevention of CMV infection in women of reproductive age is an important strategy to reduce the rate of cCMV infection. Studies have demonstrated the effectiveness of educating pregnant women on simple and inexpensive measures to prevent CMV transmission.81–83 These hygiene measures include washing hands after close contact with a child; not sharing food, utensils, cups or washcloths with a child; not kissing a child on or near the mouth; and cleaning surfaces that come in contact with a child’s urine or saliva.84 A recent study demonstrated that mothers with a child in group day care, educated on measures to prevent CMV transmission, had a significantly lower rate of CMV infection in pregnant mothers (3%) when compared to non-pregnant mothers and mothers attempting conception (42%).82 These observations were supported by an additional study where hygiene counselling on cCMV reduced maternal seroconversion rate at 36 weeks (0.19%) compared to controls prior to counselling (0.42%).83 Educating pregnant women about these hygienic precautions to reduce their risk of CMV acquisition (Table 2) has been recommended by the Centers for Disease Control and Prevention (CDC) and the American College of Obstetricians and Gynaecologists (ACOG).85,86
Table 2.
Hygienic precautions recommended by the Centers for Disease Control for pregnant women to reduce their risk of being exposed to cytomegalovirus and thus reducing the risk of fetal infection (adapted from Centers for Disease Control and Prevention86).
| Thoroughly wash hands with soap and water for 15–20 s, especially after: |
| • changing nappies |
| • feeding a young child |
| • wiping a young child’s nose or drool |
| • handling children’s toys |
| Do not share food, drinks or utensils used by young children. |
| Do not put a child’s dummy in your mouth. |
| Do not share a toothbrush with a young child. |
| Avoid contact with saliva when kissing a child. |
| Clean toys, countertops and other surfaces that come into contact with children’s urine or saliva. |
Conclusion
CMV remains a significant global health concern, with intrauterine CMV infection being the most frequent non-genetic cause of severe malformation in the newborn. Several major developments concerning CMV infection in pregnancy have been made in recent years. Sensitive and specific methods now exist for serological diagnosis of a maternal primary CMV infection, including tests to determine the avidity index of CMV IgG antibodies and the presence of IgM. To diagnose prenatal CMV infection, innovative virological tests have been developed to detect virus in the amniotic fluid, saliva or dried blood spots. Despite ongoing research, no effective therapy is currently proven to prevent or treat congenital infection. Vaccines are under development and recent studies on efficacy of administration of CMV hyperimmune globulin or antiviral drugs to pregnant women with primary CMV infection await confirmation in ongoing randomized studies. Until an effective therapy is available, education of women regarding hygiene measures that can help prevent CMV transmission is the best preventive strategy for cCMV. Obstetricians, midwives, obstetric physicians and other clinicians can lead the way in ensuring that this vital education becomes part of prenatal care.
Declaration of conflicting interest
None declared.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Guarantor
Prof. William Rawlinson
Contributorship
WvZ and WR contributed equally to the conception and design of the review. WvZ, SH, ZN and WR were responsible for the acquisition and interpretation of data. All authors contributed to drafting of the review and have provided final approval of the version to be published.
References
- 1.Manicklal S, Emery VC, Lazzarotto T, et al. The “silent” global burden of congenital cytomegalovirus. Clin Microbiol Rev 2013; 26: 86–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crough T, Khanna R. Immunobiology of human cytomegalovirus: From bench to bedside. Clin Microbiol Rev 2009; 22: 76–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cannon MJ, Hyde TB, Schmid DS. Review of cytomegalovirus shedding in bodily fluids and relevance to congenital cytomegalovirus infection. Rev Med Virol 2011; 21: 240–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adler SP. Molecular epidemiology of cytomegalovirus: A study of factors affecting transmission among children at three day-care centers. Pediatr Infect Dis J 1991; 10: 584–590. [PubMed] [Google Scholar]
- 5.Slobedman B, Cao JZ, Avdic S, et al. Human cytomegalovirus latent infection and associated viral gene expression. Future Microbiol 2010; 5: 883–900. [DOI] [PubMed] [Google Scholar]
- 6.Mocarski E, Shenk T, Pass R. Cytomegaloviruses. In: Knipe D, Howley P, Griffin D, et al. (eds). Fields virology, 5th ed Philadelphia: Lippincott Williams & Wilkins, 2007, pp. 2701–2772. [Google Scholar]
- 7.Knowles WA, Gardner SD, Fox H. A comparison of cervical cytomegalovirus (CMV) excretion in gynaecological patients and post-partum women. Arch Virol 1982; 73: 25–31. [DOI] [PubMed] [Google Scholar]
- 8.Pignatelli S, Dal Monte P, Rossini G, et al. Genetic polymorphisms among human cytomegalovirus (HCMV) wild-type strains. Rev Med Virol 2004; 14: 383–410. [DOI] [PubMed] [Google Scholar]
- 9.Renzette N, Gibson L, Bhattacharjee B, et al. Rapid intrahost evolution of human cytomegalovirus is shaped by demography and positive selection. PLoS Genet 2013; 9: e1003735–e1003735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nigro G, Anceschi MM, Cosmi EV. Clinical manifestations and abnormal laboratory findings in pregnant women with primary cytomegalovirus infection. BJOG 2003; 110: 572–577. [PubMed] [Google Scholar]
- 11.van Zuijlen W. Cytomegalovirus and ageing of the immune system: A controversial cause of ageing. Microbiology Australia 2013; 34: 157–159. [Google Scholar]
- 12.Wills MR, Mason GM, Sissons P. Adaptive cellular immunity to human cytomegalovirus. In: Reddehase MJ. (ed). Cytomegaloviruses: From molecular pathogenesis to intervention, Norfolk: Caister Academic Press, 2013, pp. 142–172. [Google Scholar]
- 13.Cannon MJ, Schmid DS, Hyde TB. Review of cytomegalovirus seroprevalence and demographic characteristics associated with infection. Rev Med Virol 2010; 20: 202–213. [DOI] [PubMed] [Google Scholar]
- 14.Basha J, Iwasenko JM, Robertson P, et al. Congenital cytomegalovirus infection is associated with high maternal socio-economic status and corresponding low maternal cytomegalovirus seropositivity. J Paediatr Child Health 2014; 50: 368–372. [DOI] [PubMed] [Google Scholar]
- 15.Hyde TB, Schmid DS, Cannon MJ. Cytomegalovirus seroconversion rates and risk factors: implications for congenital CMV. Rev Med Virol 2010; 20: 311–326. [DOI] [PubMed] [Google Scholar]
- 16.Wang C, Zhang X, Bialek S, et al. Attribution of congenital cytomegalovirus infection to primary versus non-primary maternal infection. Clin Infect Dis 2011; 52: e11–13. [DOI] [PubMed] [Google Scholar]
- 17.Mussi-Pinhata MM, Yamamoto AY, Moura Brito RM, et al. Birth prevalence and natural history of congenital cytomegalovirus infection in a highly seroimmune population. Clin Infect Dis 2009; 49: 522–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McDonagh S, Maidji E, Ma W, et al. Viral and bacterial pathogens at the maternal-fetal interface. J Infect Dis 2004; 190: 826–834. [DOI] [PubMed] [Google Scholar]
- 19.Dollard SC, Grosse SD, Ross DS. New estimates of the prevalence of neurological and sensory sequelae and mortality associated with congenital cytomegalovirus infection. Rev Med Virol 2007; 17: 355–363. [DOI] [PubMed] [Google Scholar]
- 20.Kenneson A, Cannon MJ. Review and meta-analysis of the epidemiology of congenital cytomegalovirus (CMV) infection. Rev Med Virol 2007; 17: 253–276. [DOI] [PubMed] [Google Scholar]
- 21.McMullan BJ, Palasanthiran P, Jones CA, et al. Congenital cytomegalovirus – time to diagnosis, management and clinical sequelae in Australia: opportunities for earlier identification. Med J Aust 2011; 194: 625–629. [DOI] [PubMed] [Google Scholar]
- 22.Munro SC, Hall B, Whybin LR, et al. Diagnosis of and screening for cytomegalovirus infection in pregnant women. J Clin Microbiol 2005; 43: 4713–4718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lanzieri TM, Dollard SC, Bialek SR, et al. Systematic review of the birth prevalence of congenital cytomegalovirus infection in developing countries. Int J Infect Dis 2014; 22C: 44–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zureick-Brown S, Newby H, Chou D, et al. Understanding global trends in maternal mortality. Int Perspect Sex Reprod Health 2013; 39: 32–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pass RF, Fowler KB, Boppana SB, et al. Congenital cytomegalovirus infection following first trimester maternal infection: symptoms at birth and outcome. J Clin Virol 2006; 35: 216–220. [DOI] [PubMed] [Google Scholar]
- 26.de Vries JJ, van Zwet EW, Dekker FW, et al. The apparent paradox of maternal seropositivity as a risk factor for congenital cytomegalovirus infection: A population-based prediction model. Rev Med Virol 2013; 23: 241–249. [DOI] [PubMed] [Google Scholar]
- 27.Iwasenko JM, Howard J, Arbuckle S, et al. Human cytomegalovirus infection is detected frequently in stillbirths and is associated with fetal thrombotic vasculopathy. J Infect Dis 2011; 203: 1526–1533. [DOI] [PubMed] [Google Scholar]
- 28.Pereira L, Petitt M, Fong A, et al. Intrauterine growth restriction caused by underlying congenital cytomegalovirus infection. J Infect Dis 2014; 10: 1573–1584. [DOI] [PMC free article] [PubMed]
- 29.Williams EJ, Embleton ND, Clark JE, et al. Viral infections: contributions to late fetal death, stillbirth, and infant death. J Pediatr 2013; 163: 424–428. [DOI] [PubMed] [Google Scholar]
- 30.Lorenzoni F, Lunardi S, Liumbruno A, et al. Neonatal screening for congenital cytomegalovirus infection in preterm and small for gestational age infants. J Matern Fetal Neonatal Med 2014; 15: 1589–1593. [DOI] [PubMed]
- 31.Xie F, Hu Y, Magee LA, et al. An association between cytomegalovirus infection and pre-eclampsia: A case-control study and data synthesis. Acta Obstet Gynecol Scand 2010; 89: 1162–1167. [DOI] [PubMed] [Google Scholar]
- 32.Boppana SB, Pass RF, Britt WJ, et al. Symptomatic congenital cytomegalovirus infection: neonatal morbidity and mortality. Pediatr Infect Dis J 1992; 11: 93–99. [DOI] [PubMed] [Google Scholar]
- 33.Dahl HH, Ching TY, Hutchison W, et al. Etiology and audiological outcomes at 3 years for 364 children in Australia. PLoS One 2013; 8: e59624–e59624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gabrielli L, Bonasoni MP, Lazzarotto T, et al. Histological findings in foetuses congenitally infected by cytomegalovirus. J Clin Virol 2009; 46: S16–21. [DOI] [PubMed] [Google Scholar]
- 35.Scott GM, Chow SS, Craig ME, et al. Cytomegalovirus infection during pregnancy with maternofetal transmission induces a proinflammatory cytokine bias in placenta and amniotic fluid. J Infect Dis 2012; 205: 1305–1310. [DOI] [PubMed] [Google Scholar]
- 36.Hamilton ST, Scott G, Naing Z, et al. Human cytomegalovirus-induces cytokine changes in the placenta with implications for adverse pregnancy outcomes. PLoS One 2012; 7: e52899–e52899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maidji E, Nigro G, Tabata T, et al. Antibody treatment promotes compensation for human cytomegalovirus-induced pathogenesis and a hypoxia-like condition in placentas with congenital infection. Am J Pathol 2010; 177: 1298–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cannon MJ, Westbrook K, Levis D, et al. Awareness of and behaviors related to child-to-mother transmission of cytomegalovirus. Prev Med 2012; 54: 351–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baer HR, McBride HE, Caviness AC, et al. Survey of congenital cytomegalovirus (cCMV) knowledge among medical students. J Clin Virol 2014; 60: 222–242. [DOI] [PubMed] [Google Scholar]
- 40.Jeon J, Victor M, Adler SP, et al. Knowledge and awareness of congenital cytomegalovirus among women. Infect Dis Obstet Gynecol 2006; 2006: 80383–80383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Adler SP. Cytomegalovirus and child day care: Risk factors for maternal infection. Pediatr Infect Dis J 1991; 10: 590–594. [DOI] [PubMed] [Google Scholar]
- 42.Reichman O and Miskin I. Preconception screening for cytomegalovirus: An effective preventive approach. BioMed Research International 2014; 2014: 135416. [DOI] [PMC free article] [PubMed]
- 43.Wilson J, Jungner G. Principles and practice of screening for diseases. Public Health Paper, Geneva: World Health Organization, 1968, pp. 34–34. [Google Scholar]
- 44.Walker SP, Palma-Dias R, Wood EM, et al. Cytomegalovirus in pregnancy: To screen or not to screen. BMC Pregnancy Childbirth 2013; 13: 96–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cannon MJ, Griffiths PD, Aston V, et al. Universal newborn screening for congenital CMV infection: What is the evidence of potential benefit? Rev Med Virol 2014; 5: 291–307. [DOI] [PMC free article] [PubMed]
- 46.Rahav G. Congenital cytomegalovirus infection—A question of screening. Isr Med Assoc J 2007; 9: 392–394. [PubMed] [Google Scholar]
- 47.Forsgren M. Prevention of congenital and perinatal infections. Euro Surveill 2009; 14: 2–4. [PubMed] [Google Scholar]
- 48.Rajasekariah H, Scott G, Robertson PW, et al. Improving diagnosis of primary cytomegalovirus infection in pregnant women using immunoblots. J Med Virol 2013; 85: 315–319. [DOI] [PubMed] [Google Scholar]
- 49.Stagno S, Tinker MK, Elrod C, et al. Immunoglobulin M antibodies detected by enzyme-linked immunosorbent assay and radioimmunoassay in the diagnosis of cytomegalovirus infections in pregnant women and newborn infants. J Clin Microbiol 1985; 21: 930–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.BaAlawi F, Robertson PW, Lahra M, et al. Comparison of five CMV IgM immunoassays with CMV IgG avidity for diagnosis of primary CMV infection. Pathology 2012; 44: 381–383. [DOI] [PubMed] [Google Scholar]
- 51.Enders G, Daiminger A, Bader U, et al. The value of CMV IgG avidity and immunoblot for timing the onset of primary CMV infection in pregnancy. J Clin Virol 2013; 56: 102–107. [DOI] [PubMed] [Google Scholar]
- 52.Lazzarotto T, Varani S, Spezzacatena P, et al. Maternal IgG avidity and IgM detected by blot as diagnostic tools to identify pregnant women at risk of transmitting cytomegalovirus. Viral Immunol 2000; 13: 137–141. [DOI] [PubMed] [Google Scholar]
- 53.Revello MG, Fabbri E, Furione M, et al. Role of prenatal diagnosis and counseling in the management of 735 pregnancies complicated by primary human cytomegalovirus infection: A 20-year experience. J Clin Virol 2011; 50: 303–307. [DOI] [PubMed] [Google Scholar]
- 54.Guerra B, Simonazzi G, Puccetti C, et al. Ultrasound prediction of symptomatic congenital cytomegalovirus infection. Am J Obstet Gynecol 2008; 198: 380 e381–387. [DOI] [PubMed] [Google Scholar]
- 55.Picone O, Teissier N, Cordier AG, et al. Detailed in utero ultrasound description of 30 cases of congenital cytomegalovirus infection. Prenat Diagn 2014; 34: 518–524. [DOI] [PubMed] [Google Scholar]
- 56.Benoist G, Salomon LJ, Jacquemard F, et al. The prognostic value of ultrasound abnormalities and biological parameters in blood of fetuses infected with cytomegalovirus. BJOG 2008; 115: 823–829. [DOI] [PubMed] [Google Scholar]
- 57.Yinon Y, Farine D, Yudin MH, et al. Cytomegalovirus infection in pregnancy. J Obstet Gynaecol Can 2010; 32: 348–354. [DOI] [PubMed] [Google Scholar]
- 58.Azam AZ, Vial Y, Fawer CL, et al. Prenatal diagnosis of congenital cytomegalovirus infection. Obstet Gynecol 2001; 97: 443–448. [DOI] [PubMed] [Google Scholar]
- 59.Liesnard C, Donner C, Brancart F, et al. Prenatal diagnosis of congenital cytomegalovirus infection: Prospective study of 237 pregnancies at risk. Obstet Gynecol 2000; 95: 881–888. [DOI] [PubMed] [Google Scholar]
- 60.Lazzarotto T, Varani S, Guerra B, et al. Prenatal indicators of congenital cytomegalovirus infection. J Pediatr 2000; 137: 90–95. [DOI] [PubMed] [Google Scholar]
- 61.Guerra B, Lazzarotto T, Quarta S, et al. Prenatal diagnosis of symptomatic congenital cytomegalovirus infection. Am J Obstet Gynecol 2000; 183: 476–482. [DOI] [PubMed] [Google Scholar]
- 62.Adler SP. Screening for cytomegalovirus during pregnancy. Infect Dis Obstet Gynecol 2011; 2011: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Boppana SB, Ross SA, Shimamura M, et al. Saliva polymerase-chain-reaction assay for cytomegalovirus screening in newborns. N Engl J Med 2011; 364: 2111–2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Belec L, Brogan TV. Real-time PCR-based testing of saliva for cytomegalovirus at birth. Expert Rev Anti Infect Ther 2011; 9: 1119–1124. [DOI] [PubMed] [Google Scholar]
- 65.McIver CJ, Jacques CF, Chow SS, et al. Development of multiplex PCRs for detection of common viral pathogens and agents of congenital infections. J Clin Microbiol 2005; 43: 5102–5110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Revello MG, Gerna G. Diagnosis and management of human cytomegalovirus infection in the mother, fetus, and newborn infant. Clin Microbiol Rev 2002; 15: 680–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schleiss MR. Acquisition of human cytomegalovirus infection in infants via breast milk: Natural immunization or cause for concern? Rev Med Virol 2006; 16: 73–82. [DOI] [PubMed] [Google Scholar]
- 68.Centers for Disease Control and Prevention. Cytomegalovirus and congenital Infection—Interpretation of laboratory tests, http://www.cdc.gov/cmv/clinical/lab-tests.html (accessed May 2014).
- 69.Pass RF, Zhang C, Evans A, et al. Vaccine prevention of maternal cytomegalovirus infection. N Engl J Med 2009; 360: 1191–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jacquemard F, Yamamoto M, Costa JM, et al. Maternal administration of valaciclovir in symptomatic intrauterine cytomegalovirus infection. BJOG 2007; 114: 1113–1121. [DOI] [PubMed] [Google Scholar]
- 71.Roxby AC, Atkinson C, Asbjornsdottir K, et al. Maternal valacyclovir and infant cytomegalovirus acquisition: A randomized controlled trial among HIV-infected women. PLoS One 2014; 9: e87855–e87855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hamilton ST, van Zuylen W, Naing Z, et al. Prevention of congenital cytomegalovirus by maternal and neonatal treatments: A systematic review. Reviews in Medical Virology. In press 2014. [DOI] [PubMed]
- 73.Nigro G, Adler SP, La Torre R, et al. Passive immunization during pregnancy for congenital cytomegalovirus infection. N Engl J Med 2005; 353: 1350–1362. [DOI] [PubMed] [Google Scholar]
- 74.Buxmann H, Stackelberg OM, Schlosser RL, et al. Use of cytomegalovirus hyperimmunoglobulin for prevention of congenital cytomegalovirus disease: A retrospective analysis. J Perinat Med 2012; 40: 439–446. [DOI] [PubMed] [Google Scholar]
- 75.Nigro G, Adler SP, Parruti G, et al. Immunoglobulin therapy of fetal cytomegalovirus infection occurring in the first half of pregnancy—A case-control study of the outcome in children. J Infect Dis 2012; 205: 215–227. [DOI] [PubMed] [Google Scholar]
- 76.Visentin S, Manara R, Milanese L, et al. Early primary cytomegalovirus infection in pregnancy: maternal hyperimmunoglobulin therapy improves outcomes among infants at 1 year of age. Clin Infect Dis 2012; 55: 497–503. [DOI] [PubMed] [Google Scholar]
- 77.Japanese Congenital Cytomegalovirus Infection Immunoglobulin Fetal Therapy Study Group. A trial of immunoglobulin fetal therapy for symptomatic congenital cytomegalovirus infection. J Reprod Immunol 2012; 95: 73–79. [DOI] [PubMed] [Google Scholar]
- 78.Revello MG, Lazzarotto T, Guerra B, et al. A randomized trial of hyperimmune globulin to prevent congenital cytomegalovirus. N Engl J Med 2014; 370: 1316–1326. [DOI] [PubMed] [Google Scholar]
- 79.Nigro G, Capretti I, Manganello AM, et al. Primary maternal cytomegalovirus infections during pregnancy: Association of CMV hyperimmune globulin with gestational age at birth and birth weight. J Matern Fetal Neonatal Med 2014; 1–4. [DOI] [PubMed]
- 80.Interim analysis of the Cytotec Phase III trial in congenial cytomegalovirus (CMV) infection shows clear indication of efficacy, http://www.biotest.com/ww/en/pub/investor_relations/news/newsdetails.cfm?newsID=1025191 (accessed September 2014).
- 81.Adler SP, Finney JW, Manganello AM, et al. Prevention of child-to-mother transmission of cytomegalovirus by changing behaviors: A randomized controlled trial. Pediatr Infect Dis J 1996; 15: 240–246. [DOI] [PubMed] [Google Scholar]
- 82.Adler SP, Finney JW, Manganello AM, et al. Prevention of child-to-mother transmission of cytomegalovirus among pregnant women. J Pediatr 2004; 145: 485–491. [DOI] [PubMed] [Google Scholar]
- 83.Picone O, Vauloup-Fellous C, Cordier AG, et al. A 2-year study on cytomegalovirus infection during pregnancy in a French hospital. BJOG 2009; 116: 818–823. [DOI] [PubMed] [Google Scholar]
- 84.Cannon MJ, Davis KF. Washing our hands of the congenital cytomegalovirus disease epidemic. BMC Public Health 2005; 5: 70–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.American College of Obstetrics and Gynecologists. ACOG practice bulletin. Perinatal viral and parasitic infections. Int J Gynaecol Obstet 2002; 76: 95–107. [PubMed] [Google Scholar]
- 86.Centers for Disease Control and Prevention. Cytomegalovirus and congenital infection—prevention, http://www.cdc.gov/cmv/prevention.html (accessed September 2014).