Abstract
Background
The aim of this multicentre retrospective study was to compare reverse total shoulder arthroplasty clinical outcomes with glenospheres of different diameters, designs and materials.
Methods
Between 2003 and 2008, 133 patients were divided into three groups: 60 (45%) with 36-mm standard CoCrMo (group A), 21 (16%) with 36-mm eccentric cobalt-chromium-molybdenum (CoCrMo) (group B) and 52 (39%) with 44-mm cross-linked ultra-high molecular weight polyethylene (X-UHMWPE) (group C) glenospheres. Mean (SD) follow-up was 38.7 (17.4) months. Clinical evaluation included Constant score and range of motion. Radiographic analysis included radiolucent lines, instability, loosening and assessment of scapular notching.
Results
Mean Constant score significantly increased for all groups (Wilcoxon test, p < 0.001). Group C allowed a higher and stable increase in range of motion. After 12 months and 24 months, groups C and B showed less pain than group A (Mann–Whitney U-test, p < 0.05). Group C had significantly lower scapular notching than group B (Mann–Whitney U-test, p = 0.001) and A (Mann–Whitney U-test, p = 0.009) at 12 months, 24 months and 36 months. Groups A and C presented 5 (8.3%) and 4 (7.6%) early complications, respectively.
Conclusions
The present study reported good results for all groups, although groups C and A presented better clinical outcomes, significantly lower notching and instability. A 44-mm X-UHMWPE glenosphere allowed a faster and more stable functional recovery, despite poorest pre-operative conditions. Additional long-term studies are needed to evaluate survivorship.
Keywords: Clinical results, glenospheres, reverse total shoulder arthroplasty, scapular notching, SMR
Introduction
Reverse total shoulder arthroplasty (RTSA) has proven to be a relatively recent concept that provides an effective surgical method in the management of disabling shoulder pain, where no other satisfactory option is available to restore function and limit pain.1–4 RTSA improves range of motion (ROM) in the absence of a functioning rotator cuff, as a result of its non-anatomic design medializing the centre of rotation, and thus enhancing the lever arm of the deltoid muscle.5
Early functional outcomes of RTSA have been encouraging in short- and mid-term follow-ups.2,6–9 Nevertheless, the relatively high complication rate compared with anatomic shoulder replacement remains a concern; many studies have advocated caution and recommended RTSA only to carefully selected patients.1,8,10–12 Intra-operative humeral and glenoid fractures, neurological injuries, tension-fractures of the acromion, component loosening, infections and dislocations in the postoperative period have been reported as the most frequent complications.6,10 Scapular notching, impingement of the scapular neck and component instability are common problems to be resolved.13,14
Scapular notching has been attributed to a mechanical impingement of the humeral liner against the scapular neck when the arm is fully adducted. It can be also associated with the development of an osteolytic process as a result of wear debris of the polyethylene liner.15 Scapular notching has been frequently reported after RTSA, although with different incidence values, either as completely absent16,17 or ranging from 44% to 96%.4,12,13
The aim of this retrospective study was to compare the incidence of scapular notching, ROM, pain and implant stability after RTSA with glenospheres of different design (standard and eccentric) and materials [cobalt-chromium-molybdenum (CoCrMo) and cross-linked ultra-high molecular weight polyethylene (X-UHMWPE)].
Materials and methods
Patients
Between 2003 and 2008, 133 patients (133 shoulders) underwent RTSA with the SMR system (Lima Corporate, Villanova di San Daniele, Italy), as performed by five experienced shoulder specialist surgeons at five different institutions. They were divided into three groups of evaluation according to the glenosphere design: 60 (45%) patients had a 36-mm standard CoCrMo glenosphere (group A), 21 (16%) had a 36-mm eccentric CoCrMo glenosphere (group B) and 52 (39%) had a 44-mm X-UHMWPE glenosphere (group C).
The general population included 92 (69%) females and 41 (31%) males, with a mean (SD) age of 69.2 (8.2) years (range 37 years to 87 years) at the time of surgery (Table 1). The affected side was the right shoulder in 90 (67.7%) cases and the left one in 43 (32.3%), over a population of 114 (85.7%) right-handed patients. RTSA was used as first implant in 124 (93.2%) and as revision procedure after previous failed arthroplasty in nine (6.8%). The most common indication was cuff tear arthropathy for all the three groups, followed by cuff tear in endoprostheses and secondary osteoarthritis (Table 1).
Table 1.
Distribution of patient demographics and aetiology across the three groups.
| Demographics | Group A | Group B | Group C | Total | |
|---|---|---|---|---|---|
| Gender | Female | 45 (75%) | 20 (95%) | 27 (52%) | 92 (69%) |
| Male | 15 (25%) | 1 (5%) | 25 (48%) | 41 (31%) | |
| Age (years) | Mean | 67.7 (9.2) | 68.4 (6.4) | 71.3 (7.2) | 69.2 (8.2) |
| Range | 37–85 | 58–79 | 48–87 | 37–87 | |
| Affected side | Right | 40 (66.7%) | 17 (81.0%) | 33 (63.5%) | 90 (67.7%) |
| Left | 20 (33.3%) | 4 (19.0%) | 19 (36.5%) | 43 (32.3%) | |
| Primary diagnosis | |||||
| Cuff tear arthropathy | 51 (85.0%) | 16 (76.2%) | 39 (75.0%) | 106 (79.7%) | |
| Cuff tear in endoprostheses | 5 (8.3%) | – | 4 (7.7%) | 9 (6.8%) | |
| Primary osteoarthritis | 1 (1.7%) | – | 1 (1.9%) | 2 (1.5%) | |
| Secondary osteoarthritis | 2 (3.3%) | 3 (14.3%) | 8 (15.4%) | 13 (9.8%) | |
| Rheumatoid arthritis | – | 2 (9.5%) | – | 2 (1.5%) | |
| Inveterate dislocation | 1 (1.7%) | – | – | 1 (0.7%) | |
Data presented as the mean (SD) or as indicated.
A concentric morphology of the glenoid was observed in 60 (45.5%) cases and an eccentric one in 58 (43.9%), with a similar distribution among the three groups, both for the concentric [group A: 27 (45.0%); group B: 11 (55.0%); group C: 22 (42.3%)] and for the eccentric [group A: 28 (46.7%); group B: 7 (35.0%); group C: 23 (44.2%)]. Osteolysis was limited to few cases.
Glenoid erosion was mainly classified according to Sirveaux et al.:9 a superior humeral head migration without erosion of the glenoid (E0) and a concentric erosion (E1) were present in most cases [54 (40.9%) and 49 (37.1%), respectively], followed by E2 and E3 (Table 2). The rotator cuff tear was massive in 87 (65.9%) cases, with a prevalence of Seebauer18 type 1B, 1 A and 2 A.
Table 2.
Pre-operative patient glenoid erosion and state of rotator cuff across the three groups.
| Glenoid erosion* | Group A | Group B | Group C | Total |
|---|---|---|---|---|
| E0 | 30 (50.0%) | 4 (20.0%) | 20 (38.5%) | 54 (40.9%) |
| E1 | 20 (33.3%) | 12 (60.0%) | 17 (32.7%) | 49 (37.1%) |
| E2 | 9 (15.0%) | 3 (15.0%) | 8 (15.4%) | 20 (15.2%) |
| E3 | 1 (1.7%) | 1 (5.0%) | 4 (7.7%) | 6 (4.6%) |
| State of rotator cuff§ | ||||
| Attenuated | 3 (5.0%) | – | – | 3 (2.3%) |
| Minor tear (<5 cm) | 10 (16.7%) | 13 (65.0%) | 19 (36.5%) | 42 (31.8%) |
| Massive tear (>5 cm) | 47 (78.3%) | 7 (35.0%) | 33 (63.5%) | 87 (65.9%) |
Data not acquired in four cases. §Data not acquired in one case.
Operative technique
The surgical approach was deltopectoral in 71 (53.4%) patients and deltoid split in 62 (46.6%). A general anaesthesia was performed in 105 (71.4%) patients, a general anaesthesia with locoregional in 38 (25.9%) patients and only a locoregional anaesthetic procedure in four (2.7%) patients.
Intra-operatively, the biceps tendon was found either frayed or normal in groups A and B, whereas it was always frayed in group C. The deltoid muscle was found normal in all cases. Group A showed a Goutallier19 stage 2 and 3 subscapular and infrapinatus and a Goutallier stage 3 or 4 supraspinatus. Group B had a Goutallier stage 3 subscapular, infraspinatus and supraspinatus, whereas group C showed a Goutallier stage 3 and 4 for subscapular, infraspinatus and supraspinatus.
Subscapularis and infraspinatus tendons are mandatory for a good postoperative function, stability and mid-term outcome. Muscle transfer in the event of insufficient subscapularis tendon can provide better results.2,3
Prosthetic design
The modular SMR Reverse system (Lima Corporate) includes three glenosphere designs. The 36-mm CoCrMo glenosphere has a standard concentric design and a second eccentric, both made of CoCrMo alloy and coupled with X-UHMWPE liners. The eccentric one is characterized by a 4-mm centre-of-rotation offset with respect to the metal-back axis, which allows a correct positioning and avoids inferior bone contact, independently from the metal-back position. The 44-mm glenosphere (SMR Reverse HP) has a larger diameter to improve ROM; it is characterized by an inversion of the materials to reduce polyethylene debris. It is made of X-UHMWPE and is coupled with CoCrMo liners (Figure 1). A normal reverse body of the modular system was used in 97%, the trauma version in 2% and the short type in 1%. In total, 93% of stems were cementless (normal or revision stems) and 7% were cemented. A standard or small metal-back was implanted in 65.3% and 26.5%, respectively, and a small-R version in 8.2%.
Figure 1.
SMR reverse glenosphere design and materials. (A) 36-mm standard cobalt-chromium-molybdenum (CoCrMo) glenosphere. (B) 36-mm eccentric CoCrMo glenosphere. (C) 44-mm cross-linked ultra-high molecular weight polyethylene glenosphere.
Clinical and radiographic assessment
Patients were evaluated before surgery and, postoperatively, at 3 months, 6 months, 12 months and 24 months, and then annually thereafter. Clinical evaluation was performed with the absolute and weighted Constant score (CS).20,21 Range of active movements were measured for forward flexion, external rotation with the arm at the side, external rotation in 90° of abduction and internal rotation. Radiographic evaluation included anteroposterior and axillary views to assess glenoid morphology,22 glenoid erosion9 and cuff status18 in the pre-operative analysis. At any follow-up evaluations, inferior scapular notching was classified with the Nerot grading system.23 Radiolucent lines were evaluated for the glenoid and humeral component according to location and thickness (<1 mm, 1 mm to 2 mm, >2 mm). Progression of scapular notching and radiolucent lines were evaluated using pre-operative radiographs as baseline. Radiographs were also assessed for changes in implant positioning, migration, dissociation of component and loosening. Tendons of the rotator cuff were assessed according to Goutallier classification19 from additional imaging techniques or from intra-operative observations.
Statistical analysis
Data were analyzed using SPSS, version 15.0 (SPSS Inc., Chicago, IL, USA). Descriptive statistics were computed for continuous and categorical variables. This included the mean (SD) of continuous variables and frequencies and relative frequencies of categorical factors. Determination of significant differences between the groups was realized by the Kruskal–Wallis test and the Mann–Whitney U-test. Test selection was based on evaluating the variables for normal distribution employing the Kolmogorov–Smirnov test. For categorical variables, comparisons were made using the chi-squared test (Fisher’s exact test). Comparisons within the groups were undertaken by the Friedman test and the Wilcoxon test, respectively, between the time points of clinical and radiological evaluation. All p-values resulted from two-sided statistical tests and generally values of p < 0.05 were considered statistically significant.
Results
The mean (SD) follow-up was 38.7 (17.4) months (range 24 months to 85 months). Mean absolute CS significantly increased from pre-operative to all postoperative time-points in all three groups (Wilcoxon test, p < 0.001). Although the pre-operative mean CS of group C was lower than groups A and B (Mann–Whitney U-test, p = 0.003), group C showed a mean CS percentage increase much more relevant than the other two groups (group A, CS: +31%; group B, CS: +43%; group C, CS: +50%; Mann–Whitney U-test, p < 0.001) at the last follow-up (Figure 2 and Table 3). CS was not significantly affected by age, gender, diagnosis or surgical approach (Kruskal–Wallis test, p > 0.005).
Figure 2.
Comparison of the mean Constant score among the three groups.
Table 3.
Comparison of the mean Constant score among the three groups (p < 0.001).
| Constant score | Group A | Group B | Group C |
|---|---|---|---|
| Pre-operative | 28.8 (9.7) | 26.7 (9.2) | 24.9 (11.9) |
| Last follow-up | 60.0 (9.5) | 71.4 (13.1) | 76.9 (10.9) |
| Δ% increase | +31% | +43% | +50% |
Data presented as the mean (SD) or as indicated.
Pain relief was more relevant in group C than in groups A and B (Mann–Whitney U-test, p < 0.05) at the last follow-up. Furthermore, group C showed a significant improvement of active range of movements (Mann–Whitney U-test, p < 0.05) (Table 4).
Table 4.
Comparison of the improvement in active range of movements (ROM).
| Group A | Group B | Group C | p-value | |||||
|---|---|---|---|---|---|---|---|---|
| FF,* | Pre-operative | 79.0 (25.5) | 76.8 (22.2) | 74.7 (29.3) | ||||
| Last follow-up | 115.1 (30.4) | 140.5 (18.2) | 152.8 (6.7) | A versus B p < 0.001 A versus C p < 0.001 B versus C p = 0.015 | ||||
| ER1,* | Pre-operative | 24.8 (13.0) | 20.8 (9.9) | 16.5 (11.6) | ||||
| Last follow-up | 33.7 (7.3) | 22.5 (5.5) | 29.2 (9.4) | A versus B p < 0.001 A versus C p = 0.015 B versus C p = 0.042 | ||||
| ER2,* | Pre-operative | 36.1 (15.2) | 32.9 (12.6) | 30.5 (14.2) | ||||
| Last follow-up | 58.3 (13.0) | 53.3 (8.9) | 62.8 (13.1) | A versus B p < 0.001 A versus C p = 0.015 B versus C p = 0.042 | ||||
| Group A | Group B | Group C | ||||||
| Pre- operative | Last follow-up | Pre- operative | Last follow-up | Pre- operative | Last follow-up | |||
| IR, % | Thigh | 16.9 | 44.1 | 0.0 | 0.0 | 26.0 | 0.0 | |
| Buttock | 55.9 | 41.2 | 68.4 | 5.0 | 54.0 | 0.0 | ||
| Sacroiliac joint | 18.6 | 11.8 | 21.1 | 15.0 | 2.0 | 11.1 | ||
| Waist | 8.5 | 2.9 | 10.5 | 55.0 | 14.0 | 44.4 | ||
| T12 | 0.0 | 0.0 | 0.0 | 15.0 | 4.0 | 38.9 | ||
| Shoulder blades | 0.0 | 0.0 | 0.0 | 10.0 | 0.0 | 5.6 | ||
FF, forward flexion; ER1, external rotation with the arm at the side; ER2, external rotation with 90° abduction, with the arm at the side; IR, internal rotation.
Data are the mean (SD) or as indicated.
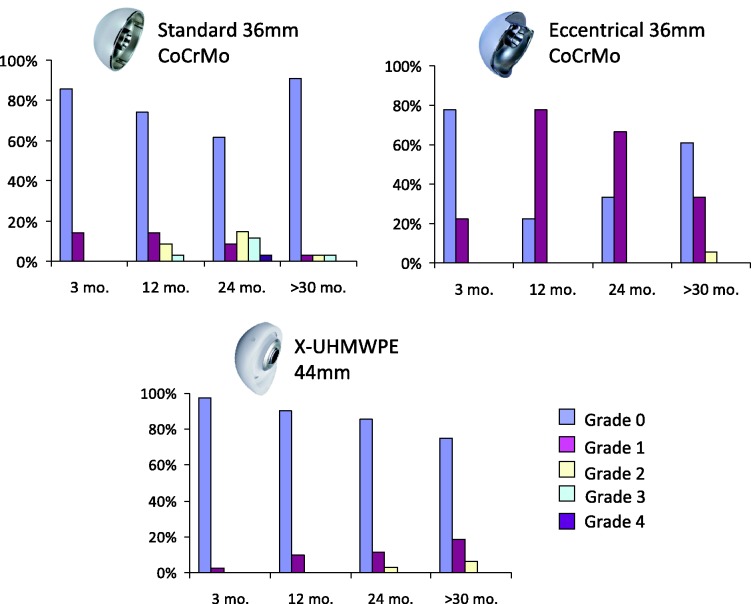
In group C, no scapular notching was observed in 85.7% and a low incidence of Nerot grades 1 and 2 (11.4% and 2.9%, respectively) was recorded after 24 months. In group B, 33.3% had no scapular notching and 66.7% had Nerot grade 1, whereas group A showed 61.8% without scapular notching, 8.8% grade 1, 14.7% grade 2, 11.8% grade 3 and 2.9% grade 4. The incidence of scapular notching was significantly lower in group C than in group B (Mann–Whitney U-test, p = 0.001) and group A (Wilcoxon test, p = 0.009) at any follow-up time-points (Figure 3). Neither progressive radiolucent lines, nor osteolysis was observed both around the metal-back and the stem at the last follow-up. No signs of instability, glenoid or stem loosening, fractures, implant failures or dissociation of components were recorded.
Figure 3.
Comparison of the incidence of scapular notching among the three groups.
Group A had five (8.3%) and group C had four (7.6%) early complications. Four cases of heterotopic ossifications were found: one in group A and three in group C. All these cases were primary prosthetic implants, although they had previous cuff repairs.
In group A, five complications occurred: a superficial wound infection as a result of Staphylococcus epidermis solved with antibiotics; a deep infection as a result of Staphylococcus aureus treated with antibiotics and surgical debridement 45 days after surgery; two early dislocations, solved with a closed reduction after 1 week and with a liner change, respectively; and one temporary axillary nerve irritation. Group B had no complications. Group C had four complications: a deep infection as a result of S. aureus that required antibiotics and surgical debris after 25 days; a superficial wound infection as a result of S. epidermidis resolved with antibiotics; a recurrent dislocation caused by a soft tissue instability, solved with a conversion to cuff tear arthropathy reconstruction 5 months after surgery; and incorrect assembly of the humeral body that required a revision 11 months later.
Discussion
The development of alternative designs of glenospheres with eccentric and larger diameters has contributed to minimizing the main limitations in RTSA, especially in terms of the incidence of scapular notching.
As a result of medialization of the centre of rotation in RTSA, the polyethylene humeral component is closer to the glenoid. This can cause impingement of the polyethylene liner against the inferior rim of the glenoid cavity and the scapular neck, with a consequent release of debris. Polyethylene liner wear can lead to joint inflammation, local osteolysis and, progressively, bone resorption, glenoid prosthetic loosening and failure. The risk of glenoid prosthetic loosening can be minimized by an inferior overhang of the glenosphere beyond its rim. This ensures that upwardly directed forces can be resisted by compression of the superior aspect of the metaglene against a solid glenoid bone; in addition, it guarantees that the central peg and fixation screws are securely anchored in the scapular bone. Inferior overhang is considered to allow a greater adduction before impingement of the humeral component on the inferior scapular neck.15
Simovitch et al. demonstrated that inferior notching is related to the position of the glenosphere on the scapula, defined with the scapular neck angle (PSNA) and the peg-glenoid rim distance (PGRD).14 A higher PSNA and a larger PGRD were correlated with an increased frequency of inferior notching. Both variables can be controlled during surgery, suggesting how pre-operative planning, proper selection of glenoid implants and precise surgical positioning of the glenoid component may assist in preventing scapular notching.
An eccentric design allows the glenosphere to extend in a lower position with respect to the inferior glenoid rim, thus limiting impingement of the humeral liner.15,24 The inversion of materials (metal liner and X-UHMWPE glenosphere) has been shown to reduce the release of polyethylene debris, although the impingement between bone and liner could still persist.24,25 A previous biomechanical study comparing the 44-mm X-UHMWPE and the 36-mm CoCrMo glenospheres (concentric and eccentric) demonstrated that the eccentric design improves ROM by allowing a higher degree of adduction, whereas glenospheres with a larger diameter (44-mm) were found to improve ROM by increasing both adduction and abduction.24–26 These findings have a major clinical significance because improved adduction may reduce mechanical impingement and hence the risk of scapular notching; they also have important implications in terms of long-term functional outcomes and durable fixation of the glenoid component.17,26,27
A causal correlation between inferior scapular notching and poor clinical outcomes has been under discussion in the literature. Simovitch et al. reported that inferior scapular notching is associated with a lower mean relative CS and a lower subjective shoulder value, as well as a lower post-operative active flexion and abduction, in a study on 77 shoulders at a mean follow-up of 44 months (range 24 months to 96 months).14 Sirveaux et al. found a negative effect of scapular notching on clinical outcome with a significant negative influence of grades 3 and 4 on the CS.9 By contrast to these results, Lévigne et al. reported that there is no clear correlation between evident inferior scapular notching and worse clinical outcome after RTSA in a consecutive clinical series of 326 patients (337 shoulders).28 At a mean follow-up of 47 months (range 24 months to 120 months), 62% had scapular notching. Notching frequency and extension were associated with the length of follow-up (normally within 14 months) and with patients’ aetiology (more frequent in the event of cuff tear arthropathy, grades 3 or 4 fatty infiltration of the infraspinatus, and narrowed acromiohumeral distance). The risk of scapular notching was observed to be correlated with a pre-operative superior orientation of glenoids, proximal humeral radiolucencies and glenoid radiolucent lines.
The objective of the present study was to compare the incidence of scapular notching and instability after RTSA with three different glenosphere designs. Outcomes were evaluated up to a maximum follow-up of 7 years.
Notching was significantly lower in group C than in groups B and A at all follow-up time-points (Figure 3). Groups C and B showed a higher increase of CS than group A, which was the group with the most elevated incidence of notching. Patients with poorest pre-operative conditions were more often treated with the 44-mm than with the 36-mm glenosphere. Despite a lower pre-operative CS, patients in group C obtained very good results, showing a more significant increment in clinical score (ΔCS + 50%) (Figure 2). The 44 mm glenosphere had a better outcome even if patients, compared with patients of the 36/36 ecc group, started with a real disabling shoulder, so lower function, higher pain level, worse ROM. This was also confirmed by an X-ray taken before surgery.
There was no difference in stability among the three groups, a fact confirming the findings of Nyffeler et al.15 Group A with a 36-mm concentric glenosphere, similar to group C with a 44-mm glenosphere, showed a low incidence of instability cases as a result of soft tissue disbalance. Radiographic analysis presented overall good results, without any signs of loosening either on the baseplate or around the screws. Similar results are also reported in a retrospective study on the survivorship of metal-back glenoid components in total shoulder replacement.29 The only case of increasing radiolucency and component instability was observed on the humeral side.
The present study displayed good outcomes after mid-term follow-up in elderly patients with significant disabling shoulder pain. Although a decreased notching percentage was recorded when the eccentric glenosphere was used, an even more significant improvement was assessed with the 44-mm glenosphere. Pain also decreased considerably and relief was constant over time.
ROM improved significantly (Table 4). Group A showed a decrease in ROM and muscular strength over 36 months. This could be attributed to an incipient loss of power in the stressed deltoid muscle in RTSA. In groups B and C, this phenomenon is present but negligible. Thus, the distalization of the centre of rotation appears also to prevent a loss of function of the deltoid muscle, providing the fibres with a better lever arm and therefore minor stress to the insertion fibres at the acromion.
The 44-mm X-UHMWPE glenosphere provided good clinical outcomes, with higher ROM, better CS and a lower incidence of instability than reported previously.30 This design also showed a decrease in notching and no incidence of loosening, confirming the evidence reported in the literature that a stable bone/metal-back baseplate interface can be achieved also in RTSA.16,29,31 Outcomes do not correlate with mechanical or polyethylene wear notching, as also reported in a previous study.32 To prevent notching, an inferior overlap of the glenosphere has been suggested as the correct implant positioning.16 There are different possibilities with respect to achieving this specific position of the centre of rotation: either lowering the baseplate itself, which may cause instability in bone fixation at the glenoid, or with an eccentric design of the glenosphere. This condition is satisfied by both the 36-mm eccentric and the 44-mm X-UHMWPE glenospheres. Down-tilt of the baseplate can be performed to improve implant longevity and clinical outcomes by decreasing stress forces on the bone/metal-back interface.
One limitation of the present study is that it is retrospective, with a mid-term follow-up. As reported, the pre-operative data for a small number of patients was not acquired in terms of glenoid morphology/erosion and state of the rotator cuff.
Conclusions
With new designs and materials for glenospheres, the SMR system contributes to overcoming the limitations of RTSA with a decreased rate of notching and instability, better survival and improved function. With inversion of the material and distalization of the centre of rotation, we observed a remarkable improvement in terms of pain relief and ROM, as well as a higher and more stable CS, without any increase in complication rate. Long-term studies are needed to confirm stable outcomes and deltoid function over time.
Declaration of conflicting interests
J. Agneskirchner, H. R. Bloch, P. Budassi and A. Castagna were consultants for Limacorporate Spa but they did not receive research support for this work. The other authors, their families or any research foundations with which they are affiliated have not received any financial payment or other benefits from any commercial entity related to the subject of this article.
Level of evidence
III – Retrospective comparative study; treatment study.
Ethical permission
No Ethics Committee approval was required because the study was retrospective and treatment with all three different devices was indicated for the encountered pathology.
References
- 1.Matsen FA, Boileau P, Walch G, Gerber C, Bicknell RT. The reverse total shoulder arthroplasty. J Bone Joint Surg Am 2007; 89: 660–7. [DOI] [PubMed] [Google Scholar]
- 2.Neer CS, Craig EV, Fukuda H. Cuff-tear arthropathy. J Bone Joint Surg Am 1983; 65: 1232–44. [PubMed] [Google Scholar]
- 3.Nicholson GP. Current concepts in reverse shoulder replacement. Curr Opin Orthop 2006; 17: 306–9. [Google Scholar]
- 4.Rockwood CA., Jr The reverse total shoulder prosthesis. The new kid on the block. J Bone Joint Surg Am 2007; 89: 233–35. [DOI] [PubMed] [Google Scholar]
- 5.Broström LA, Wallensten R, Olsson E, Anderson D. The Kessel prosthesis in total shoulder arthroplasty. A five-year experience. Clin Orthop Relat Res 1992; 277: 155–60. [PubMed] [Google Scholar]
- 6.Boileau P, Watkinson DJ, Hatzidakis AM, Hovorka I. Neer Award 2005: the Grammont reverse shoulder prosthesis: results in cuff tear arthritis, fracture sequelae, and revision arthroplasty. J Shoulder Elbow Surg 2006; 15: 527–40. [DOI] [PubMed] [Google Scholar]
- 7.De Buttet A, Bouchon Y, Capon D, Delfosse J. Grammont shoulder arthroplasty for osteoarthritis with massive rotator cuff tears: report of 71 cases. J Shoulder Elbow Surg 1997; 6: 197–197. [Google Scholar]
- 8.Frankle M, Siegal S, Pupello D, Saleem A, Mighell M, Vasey M. The reverse shoulder prosthesis for glenohumeral arthritis associated with severe rotator cuff deficiency. A minimum two-year follow-up study of sixty patients. J Bone Joint Surg Am 2005; 87: 1697–705. [DOI] [PubMed] [Google Scholar]
- 9.Sirveaux F, Favard L, Oudet D, Huquet D, Walch G, Mole D. Grammont inverted total shoulder arthroplasty in the treatment of glenohumeral osteoarthritis with massive rupture of the cuff. Results of a multicentre study of 80 shoulders. J Bone Joint Surg Br 2004; 86: 388–95. [DOI] [PubMed] [Google Scholar]
- 10.Bohsali KI, Wirth MA, Rockwood CA., Jr Complications of total shoulder arthroplasty. J Bone Joint Surg Am 2006; 88: 2279–92. [DOI] [PubMed] [Google Scholar]
- 11.Middernacht B, De Wilde L, Molé D, Favard L, Debeer P. Glenosphere disengagement: a potentially serious default in reverse shoulder surgery. Clin Orthop Relat Res 2008; 466: 892–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Werner CM, Steinmann PA, Gilbart M, Gerber C. Treatment of painful pseudoparesis due to irreparable rotator cuff dysfunction with the Delta III reverse-ball-and-socket total shoulder prosthesis. J Bone Joint Surg Am 2005; 87: 1476–86. [DOI] [PubMed] [Google Scholar]
- 13.Roberts CC, Ekelund AL, Renfree KJ, Liu PT, Chew FS. Radiologic assessment of reverse shoulder arthroplasty. Radiographics 2007; 27: 223–35. [DOI] [PubMed] [Google Scholar]
- 14.Simovitch RW, Zumstein MA, Lohri E, Helmy N, Gerber C. Predictors of scapular notching in patients managed with the Delta III reverse shoulder replacement. J Bone Joint Surg Am 2007; 89: 588–600. [DOI] [PubMed] [Google Scholar]
- 15.Nyffeler RW, Werner CML, Simmen BR, Gerber C. Analysis of a retrieved Delta III total shoulder prosthesis. J Bone Joint Surg Br 2004; 86: 1187–91. [DOI] [PubMed] [Google Scholar]
- 16.Frankle M, Levy JC, Pupello D, et al. The reverse shoulder prosthesis for glenohumeral arthritis associated with severe rotator cuff deficiency. a minimum two-year follow-up study of sixty patients surgical technique. J Bone Joint Surg Am 2006; 88(Suppl 1): 178–90. [DOI] [PubMed] [Google Scholar]
- 17.Young SW, Everts NM, Ball CM, Astley TM, Poon PC. The SMR reverse shoulder prosthesis in the treatment of cuff-deficient shoulder conditions. J Shoulder Elbow Surg 2009; 18: 622–6. [DOI] [PubMed] [Google Scholar]
- 18.Visotsky JL, Basamania C, Seebauer L, Rockwood CA, Jensen KL. Cuff tear arthropathy: pathogenesis, classification, and algorithm for treatment. J Bone Joint Surg Am 2004; 86A( Suppl 2): 35–40. [PubMed] [Google Scholar]
- 19.Goutallier D, Postel JM, Bernageau J, Lavau L, Voisin MC. Fatty muscle degeneration in cuff ruptures. Pre- and postoperative evaluation by CT scan. Clin Orthop Relat Res 1994; 304: 78–83. [PubMed] [Google Scholar]
- 20.Constant CR, Gerber C, Emery RJ, Søjbjerg JO, Gohlke F, Boileau P. A review of the Constant score: modifications and guidelines for its use. J Shoulder Elbow Surg 2008; 17: 355–61. [DOI] [PubMed] [Google Scholar]
- 21.Constant CR, Murley AHG. A clinical method of functional assessment of the shoulder. Clin Orthop Relat Res 1987; 214: 160–4. [PubMed] [Google Scholar]
- 22.Walch G, Badet R, Boulahia A, Khoury A. Morphologic study of the glenoid in primary glenohumeral osteoarthritis. J Arthroplast 1999; 14: 756–60. [DOI] [PubMed] [Google Scholar]
- 23.Valenti PH, Boutens D, Nerot C. Delta 3 reversed prosthesis for osteoarthritis with massive rotator cuff tear: long term results (> 5 years). In: Walch G, Boileau P, Molé D, eds. 2000 Prosthèses d’épaule… recul de 2 à 10 ans. Montpellier: Sauramps Médical, 2001:253–9.
- 24.Boughebri O, Duparc F, Adam JM, Valenti P. Arthroscopic dynamic analysis of scapular notching in reverse shoulder arthroplasty. Orthop Traumatol Surg Res 2011; 97: 779–84. [DOI] [PubMed] [Google Scholar]
- 25.Chou J, Malak SF, Anderson IA, Astley T, Poon PC. Biomechanical evaluation of different designs of glenospheres in the SMR reverse total shoulder prosthesis: range of motion and risk of scapular notching. J Shoulder Elbow Surg 2009; 18: 354–9. [DOI] [PubMed] [Google Scholar]
- 26.Poon PC, Chou J, Young D, Malak SF, Anderson IA. Biomechanical evaluation of different designs of glenospheres in the SMR reverse shoulder prosthesis: micromotion of the baseplate and risk of loosening. Shoulder Elbow 2010; 2: 94–9. [DOI] [PubMed] [Google Scholar]
- 27.Terragnoli F, Zattoni G, Damiani L, Cabrioli A, Li Bassi G. Treatment of proximal humeral fractures with reverse prostheses in elderly patients. J Orthopaed Traumatol 2007; 8: 71–6. [DOI] [PubMed] [Google Scholar]
- 28.Lévigne C, Boileau P, Favard L, et al. Scapular notching in reverse shoulder arthroplasty. J Shoulder Elbow Surg 2008; 17: 925–35. [DOI] [PubMed] [Google Scholar]
- 29.Castagna A, Randelli M, Garofalo R, Maradei L, Giardella A, Borroni M. Mid-term results of a metal-backed glenoid component in total shoulder replacement. J Bone Joint Surg Br 2010; 92B: 1410–15. [DOI] [PubMed] [Google Scholar]
- 30.Favard L, Lévigne C, Nerot C, Gerber C, De Wilde L, Mole D. Reverse prostheses in arthropathies with cuff tear: are survivorship and function maintained over time?. Clin Orthop Relat Res 2011; 469: 2469–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Biase CF, Delcogliano M, Borroni M, Castagna A. Reverse total shoulder arthroplasty: radiological and clinical result using an eccentric glenosphere. Musculoskelet Surg 2012; 96(Suppl 1): S27–34. [DOI] [PubMed] [Google Scholar]
- 32.Nolan BM, Ankerson E, Wiater JM. Reverse total shoulder arthroplasty improves function in cuff tear arthropathy. Clin Orthop Relat Res 2011; 469: 2476–82. [DOI] [PMC free article] [PubMed] [Google Scholar]