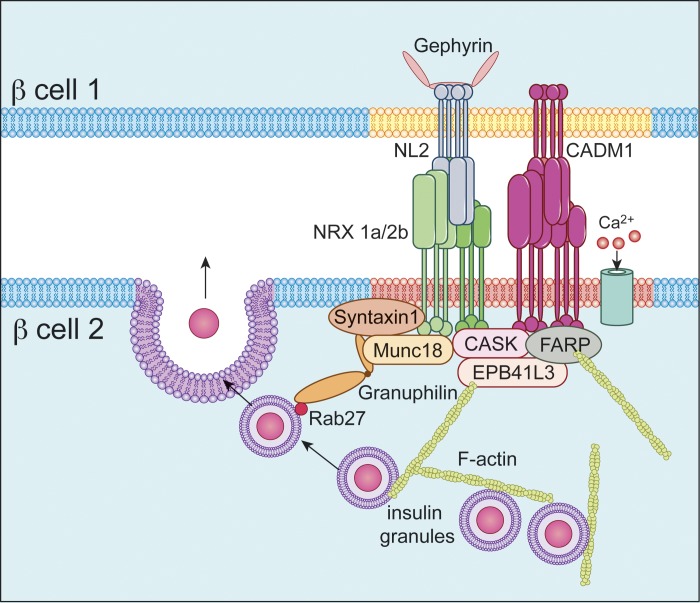
Fig. 9.
Proposed model of a site of cell-cell contact between β-cells. Transcellular CADM1-CADM1 and neuroligin (NL)-neurexin (NRX) binding interactions are depicted in the extracellular space. Also shown is the interaction of CADM1 and neurexin-1α and -2β (NRX 1a/2b) with submembrane exocytic proteins such as syntaxin-1 and with other constituents of the exocytic complex, such as Munc18, that can constrain secretion. The membrane domain in red (through which neurexin passes) and the underlying exocytic proteins represent an exocytic microdomain (excitosome). CADM1, like neurexin, binds the scaffolding protein CASK and, both directly and indirectly, other components of the submembrane insulin exocytic machinery. Granuphilin, a component of the secretory protein complex that acts to constrain insulin secretion, is also associated either directly or indirectly with CADM1 and neurexin. CADM1 also binds proteins (EPB41L3/DAL-1 and FARP) that regulate the assembly of and help anchor the cortical actin network. Neuroligin-2 passes through an as-yet-unidentified membrane domain (yellow) and binds the postsynaptic scaffolding protein gephyrin.