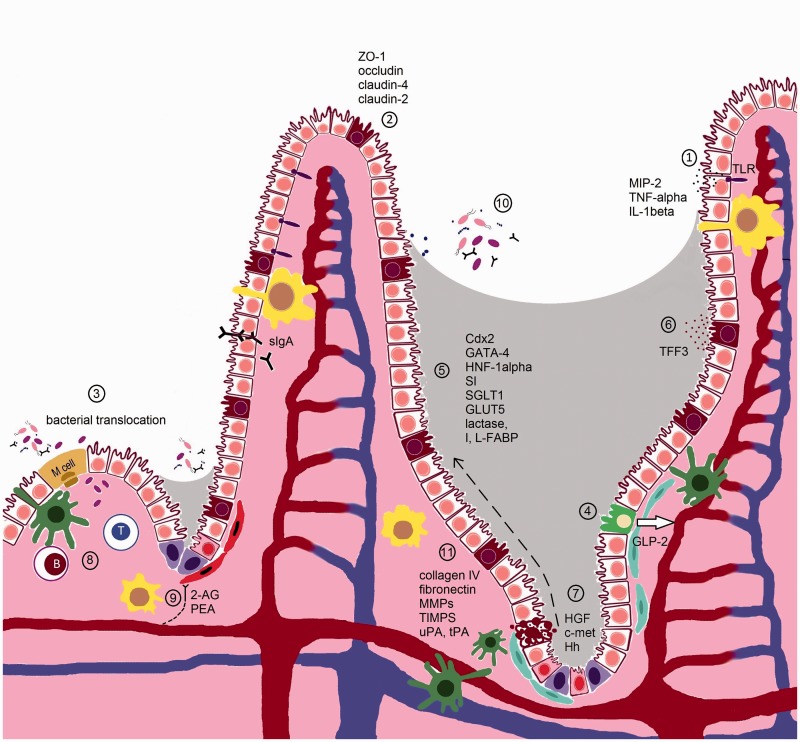
Figure 1.
Cellular and molecular targets of MTX, 5FU and irinotecan in the small intestine of the AT. In the small intestine, the barrier provided by the small IECs (white) ensures a segregation between luminal microbiota and the mucosal immune system. In the crypts, the intestinal epithelial stem cells (pink) share their niche with stromal (blue), dentritic cells (green) and macrophages (yellow), and ensure the continuous renewal of the epithelial cell layer. After differentiation, IECs migrate up the crypt–villus axis (dashed) arrow. Goblet (brown) and Paneth (purple) cells secrete mucus and antimicrobial proteins to exclude microbiota. The release of sIgA further contributes to this barrier function. M cells and goblet cells mediate transport of luminal antigens and live microbiota across the epithelial barrier to dendritic cells (green), and macrophages sample the lumen through transepithelial dendrites. Pathogen recognition receptors such as TLRs recognize conserved microbial-associated molecular motifs and pathogen-specific virulence properties. TLRs induce cytokines on ligation to signal molecules via NF-kappaB activation and the MAPK pathway. Cytotoxics MTX, 5FU, and irinotecan impair different normal epithelial intestinal functions via different mechanisms:
(1) MTX enhances the production of ROS and RNS, probably formed by phagocytes in the inflammatory regions of the small intestine, which trigger the activation of TLR4, and downstream NF-kappaB and MAPK pathways leading to elevated levels of MIP-2, TNF-alpha, and IL-1beta.
(2) MTX impairs cell–cell adhesion by destabilizing the interaction between ZO-1 and claudin-4 and altering the normal expression levels of claudin-2 (increased), claudin-4 (decreased) and occludin. Irinotecan has been shown to decrease claudin-1 and occludin expression in both the small and large intestine. This might be due to the increased expression of TNF-alpha, IL-1beta, MIP-2, and TLR4. MTX also causes the formation of RNS that probably act together with ROS in damaging small intestinal cells, either in the MLNs or the spleen.
(3) MTX increases the risk in Escherichia coli translocation and both MTX and 5FU have been shown to promote adherence of Candida albicans. Microbiota is also detected in irinotecan-treated rats.
(4) MTX stimulates the levels of GLP-2, a peptide secreted from L-type entero-endocrine cells (green) of the distal small intestine and colon, in blood and might therefore be a potential marker for MTX-induced intestinal mucositis. The disruption of basal GLP-2 R signaling seems to play a role in irinotecan-induced intestinal injury as exogenous activation of the GLP-2 receptor ameliorates gut injury.
(5) MTX downregulates the transcription factors Cdx2, GATA-4, and HNF-1alpha, which are important for intestinal development, differentiation, and gene expression. This is correlated with a decreased expression of the SI. Also enterocyte markers lactase, I-FABP, L-FABP, SGLT1, and GLUT5 are downregulated by MTX. In contrast, 5FU stimulates the activity of the Na+-dependent SGLT1 while the SGLT1 protein expression is decreased, resulting in a higher absorption of d-glucose.
(6) MTX alters the expression of TFF3, which are predominantly synthesized by the goblet cells. TFFs play an important role in preserving the integrity of the epithelial barrier.
(7) MTX, 5FU, and irinotecan induce p53-dependent apoptosis and cell cycle arrest in the epithelial stems cells of the deep crypts of the small intestine in the initial stage of mucositis. The degree of apoptosis seems to depend on the expression levels of members of the Bcl-2 family. Irinotecan, MTX, and 5FU also reduce the number of goblet cell numbers. In the mucosal regeneration phase, an upregulation of HGF and c-met can be noticed following MTX treatment while the Hh signaling seems to be important during the repair phase of the gut mucosa damage following 5FU.
(8) GALT in the intestine has been considered a centre of mucosal immunity. 5FU has been shown to reduce the number of lymphocytes at the GALT effector sites and the levels of intestinal and respiratory tract IgA levels.
(9) Endocannabinoids receptors are localized throughout the gut and predominantly located in the enteric nervous system expressed in cholinergic neurons. Receptor activation leads to relaxation in muscle cells (red) of the gut. MTX results in an overactive endocannabinoid system in the gut which might function to reduce intestinal inflammation.
(10) 5FU and irinotecan alter the microbial composition of the gut. A decrease in Clostridium spp., Lactobacillus spp., and Streptococcus spp., and an increase in Escherichia spp. could be observed in the jejunum of 5FU-treated rats. In irinotecan-treated rats, a decrease in Bacteroides spp., Lactobacillus spp., and Bifidobacterium spp can be noticed, whereas Staphylococcus spp., Clostridium spp., and E. coli are increased.
(11) Irinotecan and 5FU can cause dramatic changes in the ECM. Irinotecan results in the increase of total collagen deposits around crypts, but a decrease in collagen IV and fibronectin expression in the crypt region. Irinotecan and 5FU further alter the levels of MMP-2, -3, -9, and TIMP-1 and -2 in the jejunum. Also, the expression of the serine proteases uPA and tPA as well as PAI-1, known to play a role in MMP activation, is increased following irinotecan.