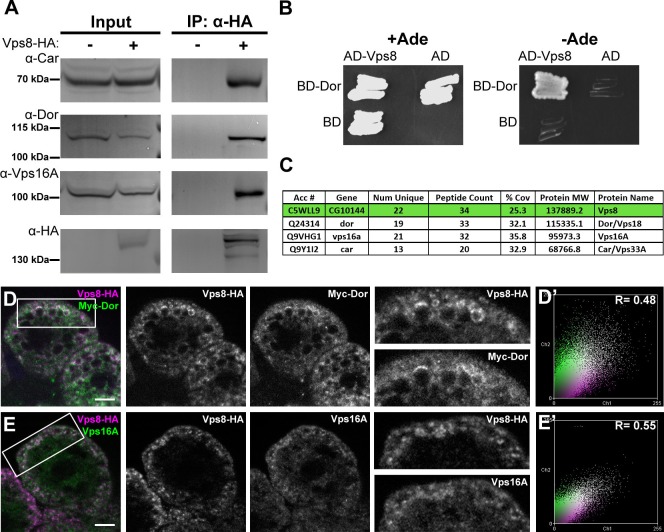
Figure 6. Vps8 forms a miniCORVET complex with the class C Vps proteins Dor/Vps18, Car/Vps33A and Vps16A.
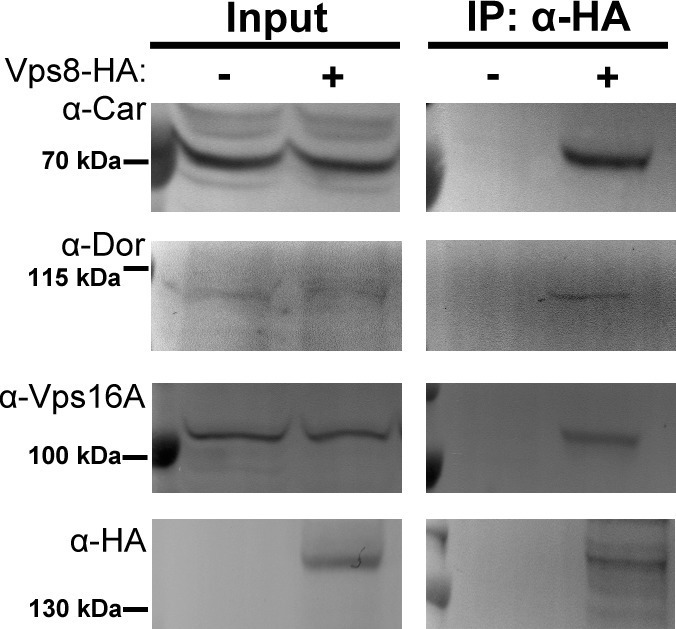
(A) Endogenous Car, Dor and Vps16A proteins coprecipitate with Vps8-HA. (B) Vps8 interacts with Dor in yeast two-hybrid experiments. Yeast colony growth on synthetic medium lacking Ade indicates a direct interaction between the two proteins. AD: Gal4 activation domain-containing vector, BD: Gal4 DNA binding domain-containing vector. (C) Summary of the mass spectrometry (MS) data from Vps8-HA immunoprecipitates. 3 Vps proteins (Dor, Car, Vps16A) coprecipitated with the bait (Vps8-HA, highlighted with green background) with high peptide count, suggesting that these 4 proteins form a stable complex. Please see Supplementary file 2 for additional proteomic data. (D,E) Vps8-HA colocalizes with myc-tagged Dor (D) or endogenous Vps16A (E). (D’, E’) Scatter plots display the intensity correlation profiles of Vps8-HA (magenta) with either myc-Dor or Vps16A (green), with Pearson correlation coefficients shown at the top. Bars: 5 µm.
Figure 6—figure supplement 1. Vps8 coprecipitates the class C Vps proteins Dor/Vps18, Car/Vps33A and Vps16A from larval lysates.