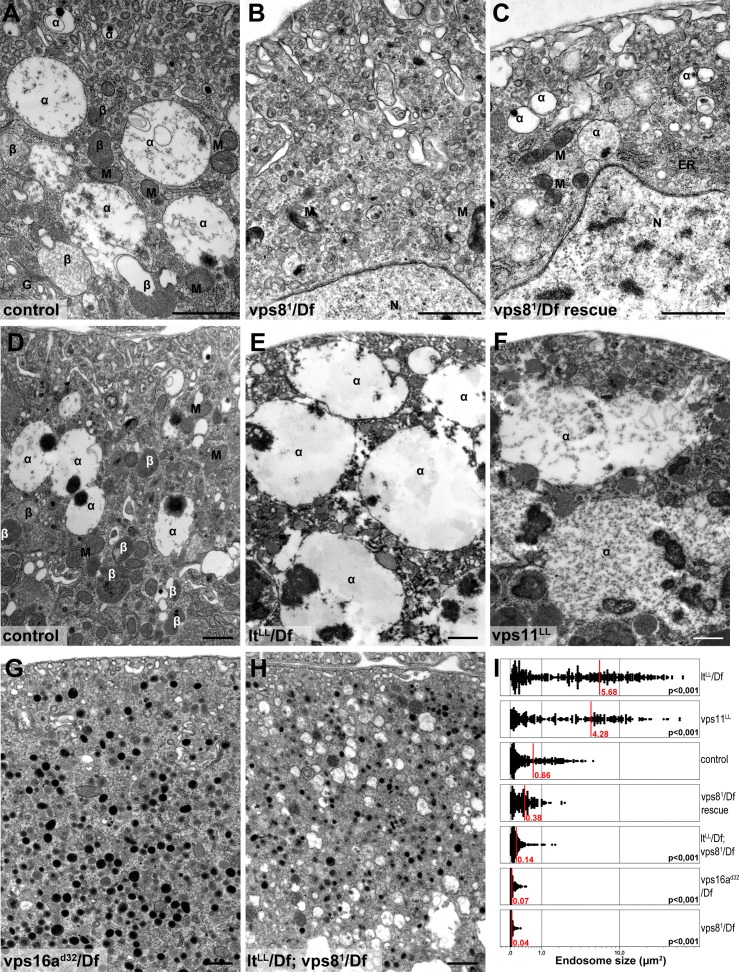
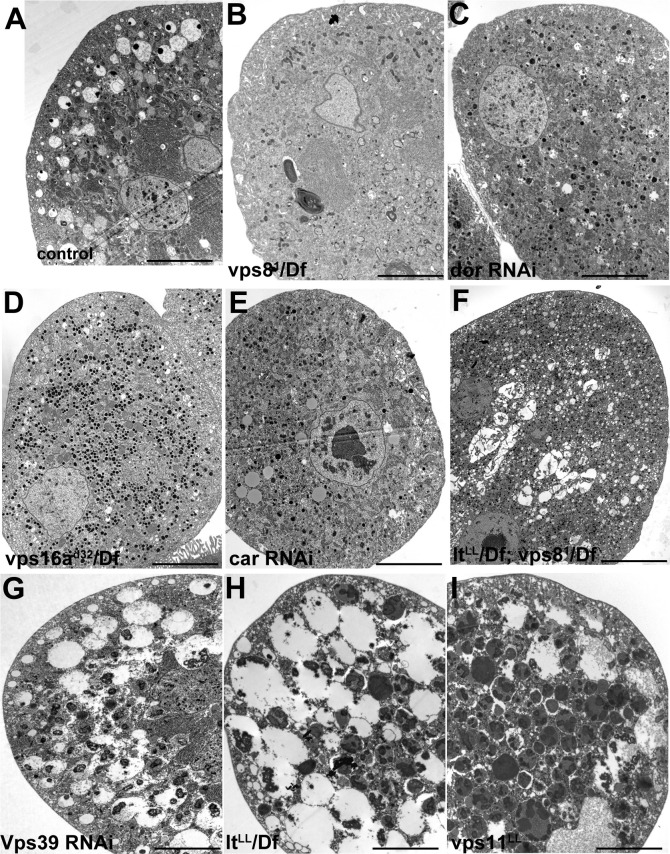
Figure 8. Loss of miniCORVET leads to fragmentation of endosomes, unlike HOPS defects.
Vps81 mutant (B) garland nephrocytes lack normal sized α-vacuoles and only small vesicles can be found, unlike in control (A,D) or rescued (C) cells. In contrast, nephrocytes lacking either the HOPS specific subunit Lt/Vps41 (E) or Vps11 (F) contain enlarged α-vacuoles, which often have multiple cores. The simultaneous loss of both miniCORVET and HOPS function in vps16A single (G) or lt and vps8 double mutant cells (H) also results in fragmented, small α-vacuoles similar to vps8 single mutants. Note that these nephrocytes also contain numerous small electron-dense organelles, which may represent a Golgi-derived pre-lysosomal compartment. N: nucleus, M: mitochondria, ER: endoplasmic reticulum. (I) Quantification of data from panels (A–G). The size of each endosome is represented as a single punctum on the dot plots. Red lines and numbers show the mean endosome area in µm2. Bars: 1 µm. Please see Figure 8—figure supplement 1. for additional data.