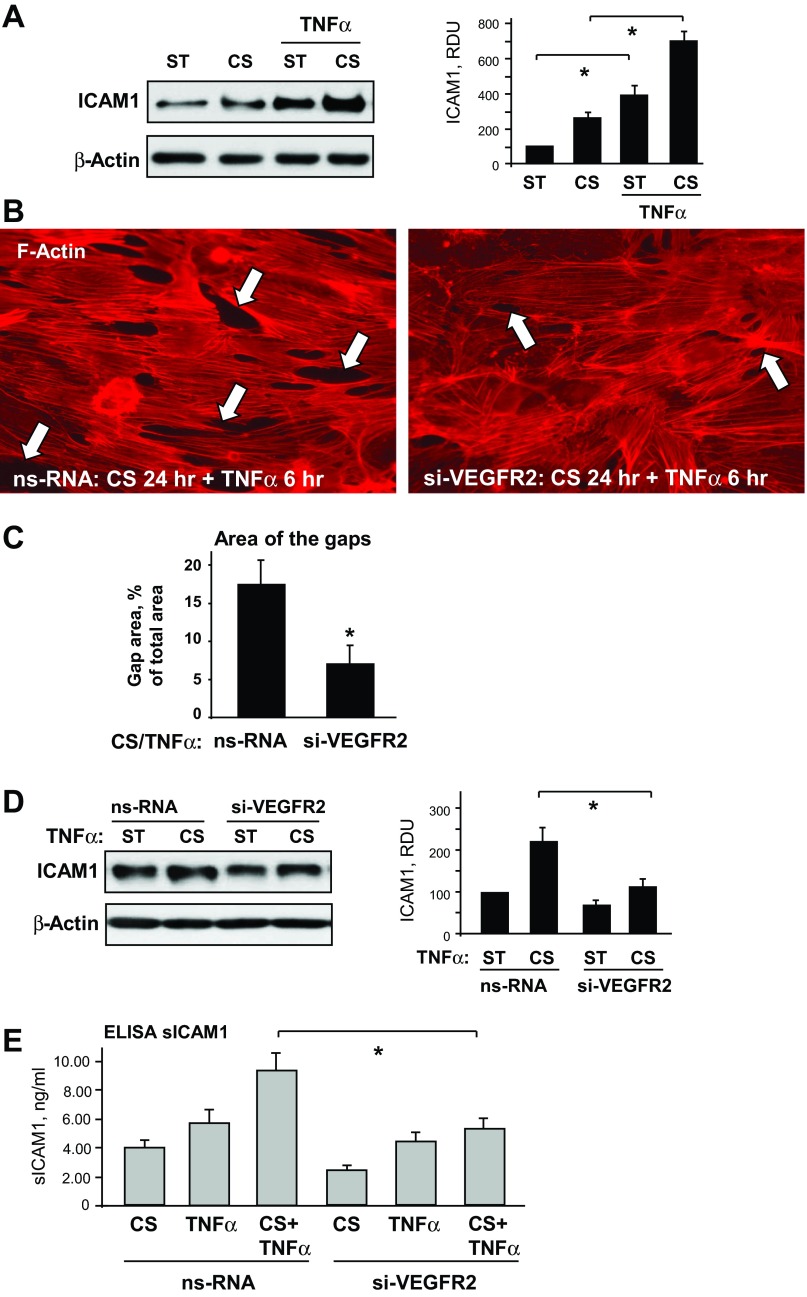
Fig. 5.
Role of VEGFR2 signaling in the 2-hit model of EC dysfunction. A: HPAECs were subjected to 18% CS for 24 h followed by TNF-α (20 ng/ml, 4 h) stimulation. Control cells were left under static conditions. ICAM1 expression was analyzed in control and stretched cells. β-Actin was used as a normalization control; n = 3; *P < 0.05 vs. static. B and C: ECs were transfected with VEGFR2-specific RNA or ns-RNA, followed by application of 18% CS for 24 h and TNF-α stimulation. Cytoskeletal remodeling in control and stretched ECs was examined by immunofluorescence staining for F-actin; paracellular gaps are marked by arrows (B). Quantitative analysis was performed of paracellular gap formation in control and VEGFR2-depleted ECs. Data are expressed as means ± SD; n = 3, *P < 0.05 vs. ns-RNA (C). D: effect of VEGFR2 knockdown on CS-induced ICAM1 expression was analyzed in static and stretched cells (18% CS, 24 h) stimulated with TNF-α (20 ng/ml, 4 h). β-Actin was used as a normalization control; n = 3; *P < 0.05 vs. ns-RNA. E: sICAM1 content in the culture media from control and VEGFR2-depleted cells was determined in stretch (18% CS, 24 h) and/or TNF-α-stimulated samples (20 ng/ml, 4 h) using ELISA assay; n = 4, *P < 0.05 vs. ns-RNA.