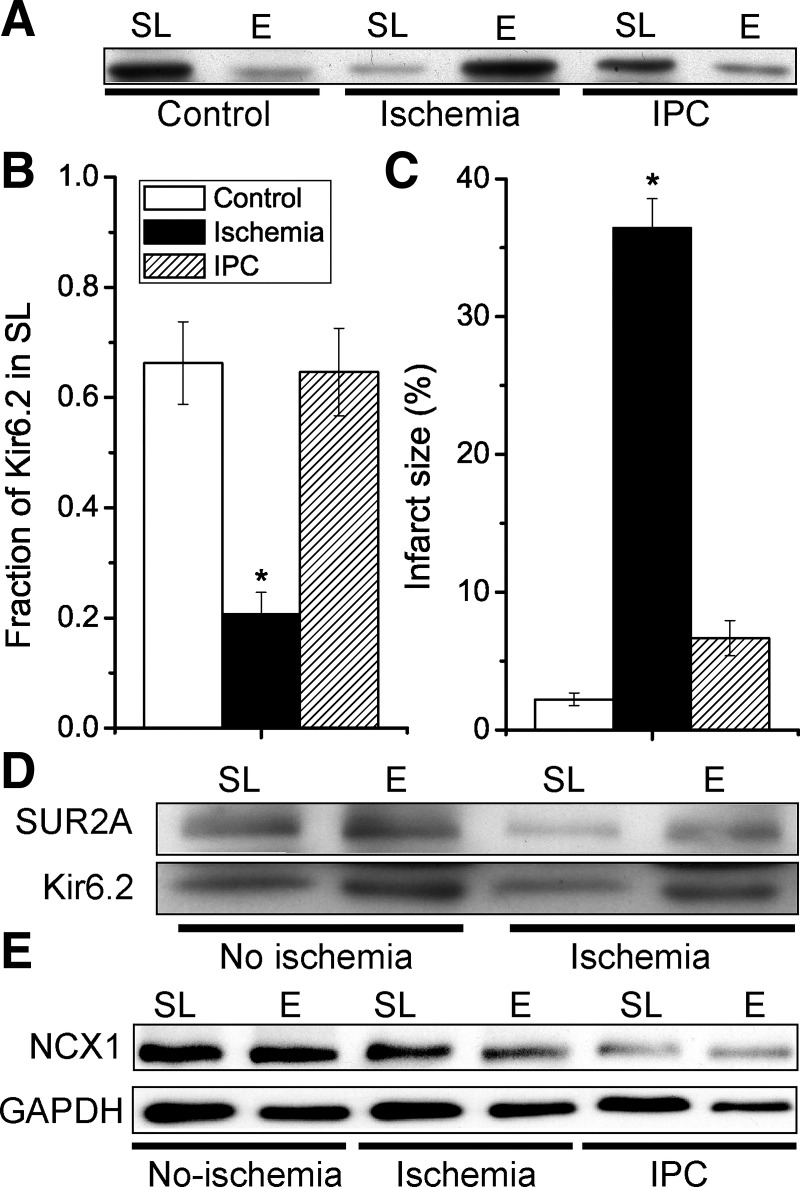
Fig. 2.
Ischemic preconditioning not only limits infarct size but also prevents ischemia-induced internalization of the Kir6.2 KATP channel subunit. Isolated mouse hearts were made globally ischemic for 30 min, either with (IPC) a 3 × 5 min IPC protocol or without (ischemia group). A time-matched control normoxic group was also included. A: Western blot with an anti-Kir6.2 antibody of sarcolemmal (SL; 0–10% Optiprep) and endosomal (E; 10–20% Optiprep) membranes for the various groups. B: summary data of sarcolemmal Kir6.2 (as a fraction of the total amount; SL/[SL + E]) for the control (n = 9), ischemia (n = 9), and IPC (n = 7). C: in a different set of hearts, infarct sizes were determined by TTC staining; control (n = 8), ischemia (n = 12), and IPC (n = 7). *P < 0.05 vs. the control group. D: redistribution of Kir6.2 from sarcolemmal to endosomal membrane fractions with ischemia was also observed with SUR2A. E: internalization of Kir6.2 during ischemia is not a general phenomenon. Sarcolemmal and endosomal membranes were prepared from mouse hearts with normoxic perfusion (control), after 30-min global ischemia (ischemia) or with 3 × 5 min ischemia before a 30-min index ischemia (IPC). Immunoblotting was performed with antibodies against Na+/Ca2+ exchanger (NCX)1 and GAPDH. Data are representative of n = 3 experiments.