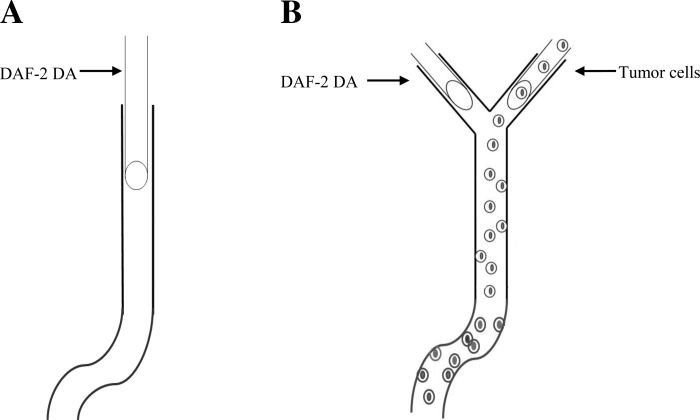
Fig. 1.
Schematic showing the technique used to measure nitric oxide (NO) production in the endothelial cells forming the microvessel wall with and without tumor cell perfusion. A: without tumor cell perfusion. A postcapillary venule of 500∼1000 μm long with straight and curved portions is cannulated with a micropipette and loaded with 4,5-diaminofluorescein diacetate (DAF-2 DA; 5 μM) in 1% BSA-Ringer solution for 45 min at a reduced perfusion rate of ∼300 μm/s; DAF-2 NO images of the microvessel are then taken every 5 min up to 30 min under a perfusion rate of ∼1,000 μm/s (normal flow) or ∼300 μm/s (reduced flow). B: with tumor cell perfusion. A postcapillary venule of 500∼1,000 μm long with straight and curved portions and with a Y-branch at its upstream is found first. One branch is cannulated and loaded with DAF-2 DA (5 μM) in 1% BSA-Ringer solution for 45 min at a reduced perfusion rate of ∼300 μm/s while another branch is occluded by a glass pipette blocker. At the end of 45 min, the branch vessel for DAF-2 DA loading is blocked and another branch is cannulated and perfused with cell tracker red-labeled tumor cells in 1% BSA-Ringer solution at a normal or reduced rate. Every 5 min up to 30 min, DAF-2 NO and tumor cell adhesion images of the vessel are then taken alternately by switching the light to green (excitation 490/emission 520 nm] for DAF-2 NO and to red (excitation 577/emission 602 nm) for tumor cells.