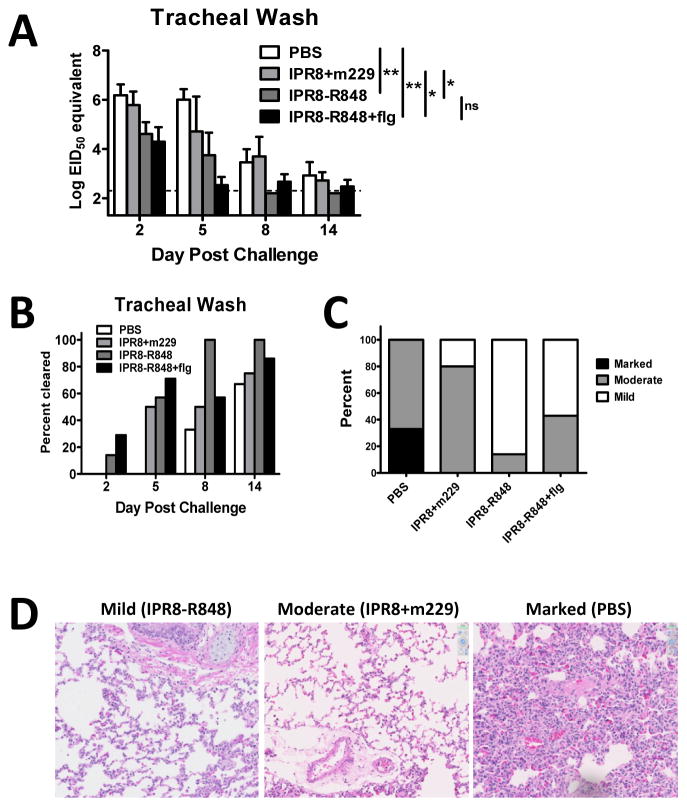
Figure 6. Vaccination with R848 conjugated IPR8 results in recall responses that are more protective.
(A) Virus load in the trachea following virus challenge of vaccinated animals was assessed by RT-PCR. Virus in the trachea of IPR8-R848 vaccinated infants was reduced following challenge compared to m229 vaccinated and PBS animals. Data shown are EID50 equivalents calculated based on known concentrations of influenza virus. Significance was determined using a repeated measures mixed model fit. *p<0.05, **p<0.01. (B) Data represent the percentage of animals in which virus was below the limit of detection in the trachea at each indicated time following challenge. IPR8-R848 vaccinated animals show earlier clearance compared to other vaccine groups. (C) The severity of lung pathology was determined in a blinded fashion by a board certified veterinary pathologist. Pathology was assigned as mild, moderate, or marked for each animal. Infants with mild pathology exhibited alveoli that were open and regularly contained few inflammatory cells that were mostly macrophages with few neutrophils. Alveolar septa were mildly thickened in some areas. Connective tissue fibers around vessels were separated by clear space, consistent with edema. Moderate changes were characterized by slight thickening of the alveolar walls due to fibrin and prominent type 2 pneumocytes (type 2 pneumocyte hyperplasia), more prominent perivascular edema and regular intra-alveolar macrophages. Severe interstitial pneumonia was characterized by diffuse hypercellularity, obscuring the small airways. Whereas the majority of animals vaccinated with IPR8+m229 had moderate lung pathology, the vast majority of animals receiving IPR8-R848 exhibited mild disease. (D) Representative sections for mild, moderate, and marked changes in the lungs of challenged animals are shown.