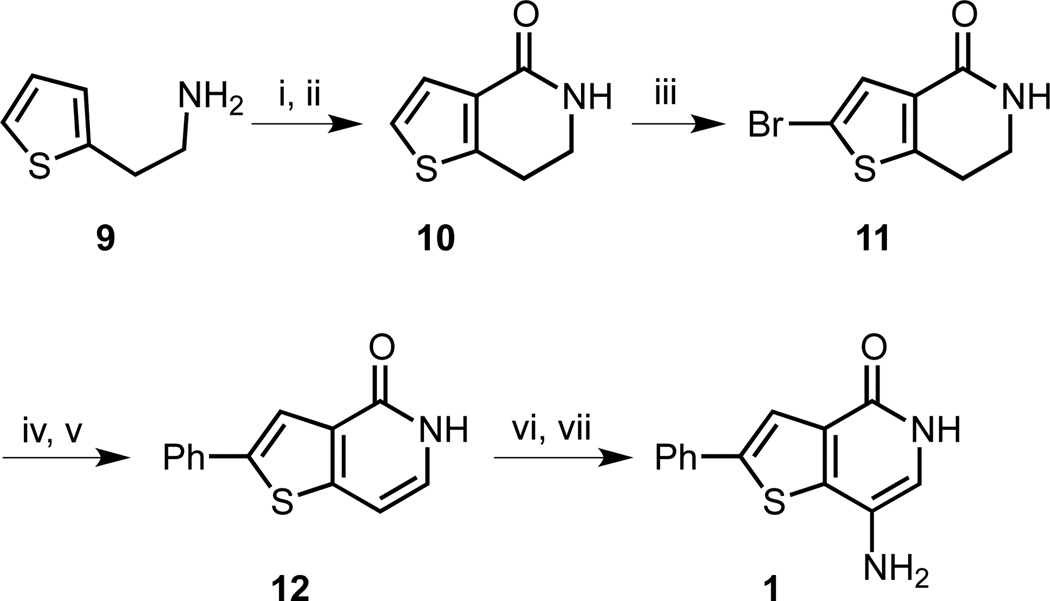
Scheme 2.
Second generation synthesis of thienopyridone 1. Reagents and conditions: (i) CO(OCCl3)2, NaHCO3, CH2Cl2/H2O, 0 °C, 5 h; (ii) FeCl3, CH2Cl2, 50 °C, 40 min, 71% (2 steps); (iii) Br2, AcOH, room temperature, 12 h, 75%; (iv) Pd(PPh3)4, PhB(OH)2, Na2CO3, dioxane/H2O, 90 °C, 24 h; (v) DDQ, dioxane, 101 °C, 2.5 d, 48% (2 steps); (vi) HNO3, AcOH, room temperature, 15.5 h; (vii) 10% Pd/C (17 mol%), H2 (1 atm), EtOH, room temperature, 5 h, 19% (2 steps).