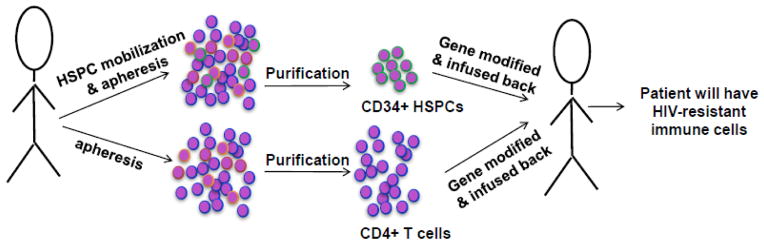
Fig 5. Schematic of potential RNAi therapy strategy for humans.
Two approaches can be used for human therapy. Either autologous CD4 T cells (purified from PBMC obtained by apheresis) or CD34+ HSPC (purified from bone marrow or blood after mobilization by GM-CSF treatment) can be in vitro modified to express shRNA (generally by transduction with lentivirus encoding CCR5 and antiviral shRNAs) and reinfused into the patients. Modified CD4 T cells provide a one-time source of HIV-1 resistant T cells. HSPC modification is expected to result in the continuous generation of HIV-1 resistant progeny T cells and macrophages.