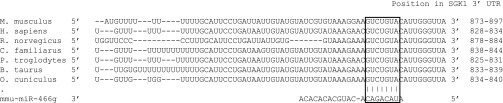Abstract
Micro-RNAs (miRNAs) are noncoding RNAs that bind target mRNA transcripts and modulate gene expression. In the cortical collecting duct (CCD), aldosterone stimulates the expression of genes that increase activity of the epithelial sodium channel (ENaC); in the early phase of aldosterone induction, one such gene is serum and glucocorticoid regulated kinase 1 (SGK1). We hypothesized that aldosterone regulates the expression of miRNAs in the early phase of induction to control the expression of target genes that stimulate ENaC activity. We treated mpkCCDc14 cells with aldosterone or vehicle for 1 h and used a miRNA microarray to analyze differential miRNA expression. We identified miR-466g as a miRNA that decreased by 57% after 1 h of aldosterone treatment. Moreover, we identified a putative miR-466g binding site in the 3′-untranslated region of SGK1. We constructed an SGK1 3′-untranslated region luciferase reporter and found that cotransfection of miR-466g suppressed luciferase activity in human embryonic kidney-293 cells in a dose-dependent manner. Deletion or introduction of point mutations that disrupt the miR-466g target site attenuated miR-466g-directed suppression of luciferase activity. Finally, we generated stably transduced mpkCCDc14 cell lines overexpressing miR-466g. Cells overexpressing miR-466g demonstrated 12.9-fold lower level of SGK1 mRNA compared with control cells after 6 h of aldosterone induction; moreover, cells overexpressing miR-466g exhibited 25% decrease in amiloride-sensitive current after 6 h of aldosterone induction and complete loss of amiloride-sensitive current after 24 h of aldosterone induction. Our findings implicate miR-466g as a novel early-phase aldosterone responsive miRNA that regulates SGK1 and ENaC in CCD cells.
Keywords: aldosterone, SGK1, ENaC, miRNA, cortical collecting duct
micro-rnas (mirnas) are a class of small noncoding RNAs that posttranscriptionally regulate gene expression by binding to target mRNA transcripts and repressing gene expression (4). miRNAs select their targets by base-pairing with sites in the 3′-untranslated region (3′-UTR) of target mRNAs (5). By either increasing mRNA degradation or inhibiting protein translation, miRNAs modulate and fine-tune the expression of genes that regulate a wide array of biological processes.
Aldosterone regulates extracellular fluid volume and blood pressure primarily by stimulating epithelial sodium channel (ENaC) activity in the cortical collecting duct (CCD). Aldosterone binds to mineralocorticoid receptors expressed in the CCD and stimulates Na+ transport in two distinct phases: early (within 2.5 h of hormone addition) and late (after 2.5 h) (36). In the early phase of induction, aldosterone activates preexisting ENaC channels through transcriptional regulation of proteins that stimulate ENaC activity. Serum and glucocorticoid regulated kinase 1 (SGK1) is now recognized as a prototypic early-response gene and a key mediator of the stimulatory effects of aldosterone on ENaC activity in CCD cells (1, 8, 17, 27).
Regulatory networks involving nuclear receptor and miRNA pathways are emerging as logical control points for controlling cell phenotypes in diverse systems (9, 16, 30, 31). Aldosterone, by activating mineralocorticoid receptors, may similarly signal through miRNAs to control expression of genes whose products regulate ENaC activity in the CCD. Recent studies have examined aldosterone-induced changes in miRNA expression in the collecting duct; however, these studies focused on changes in miRNA expression during the late phase of aldosterone induction (12, 13, 20). To date, no studies have identified early-phase, aldosterone-regulated miRNAs in the CCD; nor have any studies manipulated expression of specific miRNAs in CCD cells to examine functional effects of early-phase, aldosterone-regulated miRNAs on Na+ transport. A clear understanding of the mRNA targets and gene networks that are regulated by aldosterone-regulated miRNAs in the CCD is thus still lacking.
In this study, we hypothesized that aldosterone regulates the expression of a set of miRNAs in the early phase of induction to control expression of target genes that stimulate ENaC activity. We used the mpkCCDc14 cell line, a well-established model for CCD epithelia that exhibits classic electrophysiological features of the CCD (6), to identify and characterize early-phase, aldosterone-regulated miRNAs. We treated polarized mpkCCDc14 cells with aldosterone or vehicle for 1 h and used a miRNA microarray to analyze differential miRNA expression. Here we identify mmu-miR-466g (miR-466g) as an early-phase, aldosterone-responsive miRNA that regulates SGK1 mRNA expression and ENaC activity in CCD cells.
METHODS
Cell culture.
Immortalized mouse kidney CCD (mpkCCDc14) cells were kindly provided by Dr. Alain Vandewalle (Institut National de la Santé et de la Recherche Médicale) and maintained as previously described (34). mpkCCDc14 cells were seeded onto 24-mm permeable supports (0.4-μm pore size, Costar, Corning, NY) and grown in defined medium until transepithelial resistance reached values between 800 and 1,200 Ω·cm2, as measured with an EVOM2 “chopstick” voltmeter (World Precision Instruments, Sarasota, FL). Cells were then switched to supplement and serum-free media for 48 h before treatment with aldosterone (10−6 M) or 100% ethanol vehicle.
miRNA microarray.
Polarized mpkCCDc14 cells were treated with aldosterone (10−6 M) or ethanol vehicle for 1 h. Cells were washed with PBS, and RNA was isolated using the miRNeasy Mini Kit (Qiagen, Valencia, CA) per manufacturer's instructions. Total RNA was quantified using a Nanodrop Spectrophotometer (Thermo Scientific, Wilmington, DE). Expression of 609 mouse miRNAs was evaluated by using the Affymetrix miRNA Array (Affymetrix, Santa Clara, CA) by the Stanford University Protein and Nucleic Acid Facility. Comparison between expression of miRNAs in aldosterone and vehicle treatment groups was determined with the Partek Genomics Suite (Partek, St. Louis, MO). Differential miRNA expression was defined as >1.5-fold change in miRNA expression between aldosterone and vehicle treatment groups.
Quantitative PCR.
For analysis of miR-466g expression in mpkCCDc14 cells, RNA was isolated using the miRNeasy mini kit (Qiagen, Germantown, MD) and reverse transcribed using the miRNA Reverse Transcription Kit (Applied Biosystems, Carlsbad, CA). miR-466g expression was evaluated with TaqMan RT primers specific to miR-466g, FAM-labeled TaqMan primers specific to miR-466g, and the TaqMan Universal PCR Master Mix system, according to the manufacturer's protocol (Applied Biosystems). Reactions were amplified using the Applied Biosystems Prism 7900HT Fast Real-Time PCR system. Results were expressed as 2−ΔΔCt values, with ΔCt = CtmiR − Ctsno202 (29). Expression of miR-466g was normalized to expression of the control miRNA sno-202. The small nucleolar RNA sno-202 was used as a loading control because expression of this miRNA is unchanged with aldosterone stimulation in mouse kidney (13).
For analysis of SGK1 mRNA levels in mpkCCDc14 cells, RNA was isolated using the RNeasy mini kit (Qiagen, Germantown, MD) and reverse transcribed using the High Capacity RNA-to-cDNA Kit (Applied Biosystems). SGK1 expression was evaluated with TaqMan primers specific to mouse SGK1 and the TaqMan Universal MasterMix II Master Mix System (Applied Biosystems). Expression of SGK1 mRNA was normalized to expression of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNA, and relative expression of SGK1 in aldosterone-treated cells was presented as fold change from untreated cells.
Computational analysis.
Prediction of mRNA targets of miR-466g was assessed by the following online algorithms: TargetScan (http://www.targetscan.org), miRanda (http://www.microrna.org/microrna/home.do), and PicTar (http://pictar.mdc-berlin.de). Conservation of miR-466 target sequences in the 3′-UTR of SGK1 of different species was determined by using the NCBI GenBank, and nucleotide sequences were aligned using the T-Coffee Multiple Sequence Alignment Program (http://tcoffee.crg.cat).
Generation of luciferase reporter constructs.
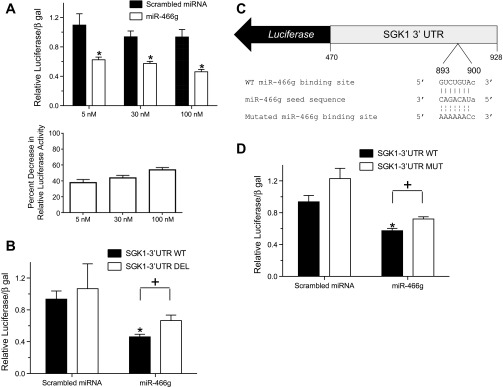
An SGK1-3′-UTR luciferase reporter plasmid [SGK1-3′-UTR wild type (WT)] was generated by cloning 459 bp of the mouse SGK1 3′-UTR sequence from nucleotides 472–930 (after the SGK1 stop codon) into the pMIR-REPORT miRNA Expression Reporter Vector (Life Technologies). An SGK1-3′-UTR deletion construct (SGK1-3′-UTR DEL) was generated by cloning a 230-bp fragment of the SGK1 3′-UTR from nucleotides 472–701, which is missing the miR-466g target sequence, into the pMIR-REPORT miRNA Expression Reporter Vector. The SGK1-3′-UTR MUT construct was generated by introducing serial point mutations (see Fig. 4C) into SGK1-3′-UTR WT with the QuikChange II Site-Directed Mutagenesis Kit (Agilent, Santa Clara, CA) per manufacturer's instructions.
Fig. 4.
miR-466g interacts with its target sequence in the 3′-UTR of SGK1. A: HEK-293 cells were cotransfected with a luciferase reporter containing the 3′-UTR of SGK1 (SGK1-3′-UTR WT) and increasing doses of a scrambled miRNA or miR-466g. B: HEK-293 cells were cotransfected with SGK1 3′-UTR WT or a luciferase reporter in which the putative miR-466g binding site in the SGK1 3′-UTR was deleted (SGK1-3′-UTR DEL) and either scrambled miRNA or miR-466g (10−7 M). C: schematic of a luciferase reporter in which serial point mutations have been introduced into the putative miR-466g seed binding site (nucleotides 893–900) of the 3′-UTR of SGK1 (SGK1-3′-UTR MUT). D: HEK-293 cells were cotransfected with SGK1-3′-UTR WT or SGK1-3′-UTR MUT and either scrambled miRNA or miR-466g (3.0 × 10−8 M). In all experiments, luciferase activity was first normalized to β-galactosidase activity and whole cell protein concentration and then normalized to background luciferase/β-galactosidase activity in cells transfected with luciferase vector. Values are means ± SE, from at least 5 independent experiments. *P < 0.05 between cells transfected with scrambled miRNA and miR-466g. +P < 0.05 between cells transfected with SGK1-3′-UTR and either SGK1-3′-UTR DEL or SGK1-3′-UTR MUT.
Transient transfection luciferase assay.
Human embryonic kidney-293 cells (passages 5–20) were transfected in 24-well cell culture plates with 37.5 ng of luciferase reporter plasmid, 25 ng β-galactosidase control plasmid, and 5–100 × 10−9 M of scrambled or precursor miR-466g (Applied Biosystems). Lipofectamine 2000 (Invitrogen, Carlsbad, CA) was used as a transfection reagent in all experiments. Forty-eight hours after transfection, cells were harvested, and protein lysates were assessed for luciferase and β-galactosidase activities with a Femtomaster FB 12 Luminometer (Zylux, Huntsville, AL) and a chemiluminescence reporter assay system (Dual Light Luciferase Assay system, Applied Biosystems). Luciferase activity was normalized to β-galactosidase activity and whole cell protein concentration and then normalized to background luciferase/β-galactosidase activity/whole cell protein concentration in cells transfected with luciferase vector.
Stable transduction of mpkCCDc14 cells.
The SMARTchoice Promoter Selection plate (GE Healthcare, Sunnyvale, CA) was used to identify the mouse cytomegalovirus promoter as the most active promoter at a multiplicity of infection level 4 for driving lentiviral short hairpin RNA expression in mpkCCDc14 cells. mpkCCDc14 cells (passage 16) were plated in 96-well cell culture dishes and transduced with SMARTchoice mouse lentiviral mmu-miR-466g shMIMIC mouse cytomegalovirus/TurboGFP or SMARTvector 2.0 Mouse GAPDH mCMV-TurboGFP control particles in serum-free media supplemented with DEAE-dextran (1 μg/ml). Forty-eight hours after transduction, cells were expanded into T-75 cm2 flasks with media containing puromycin to select for cells with stably integrated short hairpin RNA. Overexpression of mmu-miR-466g was verified by quantitative PCR as described above. For all aldosterone time course experiments, stably transduced cells between passages 4 and 8 were used.
Aldosterone time course experiments.
Stably transduced mpkCCDc14 cells were seeded onto permeable supports and grown in defined medium until transepithelial resistance reached values between 800 and 1,200 Ω·cm2. Before aldosterone treatment, the media was replaced with supplement and serum-free media for 48 h. Equivalent current (potential difference/resistance) was measured using an EVOM2 voltmeter at 1, 3, 6, and 24 h after cells were treated with aldosterone (10−6 M) or ethanol vehicle.
Statistical analysis.
Statistical analysis for comparison between different treatment groups was performed using two-tailed Student's t-test. Differences were considered to be significant at P values < 0.05. Findings are reported as mean values ± SE. Prism 5 Software (GraphPad, La Jolla, CA) was used to perform graphical analysis.
RESULTS
Aldosterone rapidly modulates expression of a set of miRNAs in mpkCCDc14 cells.
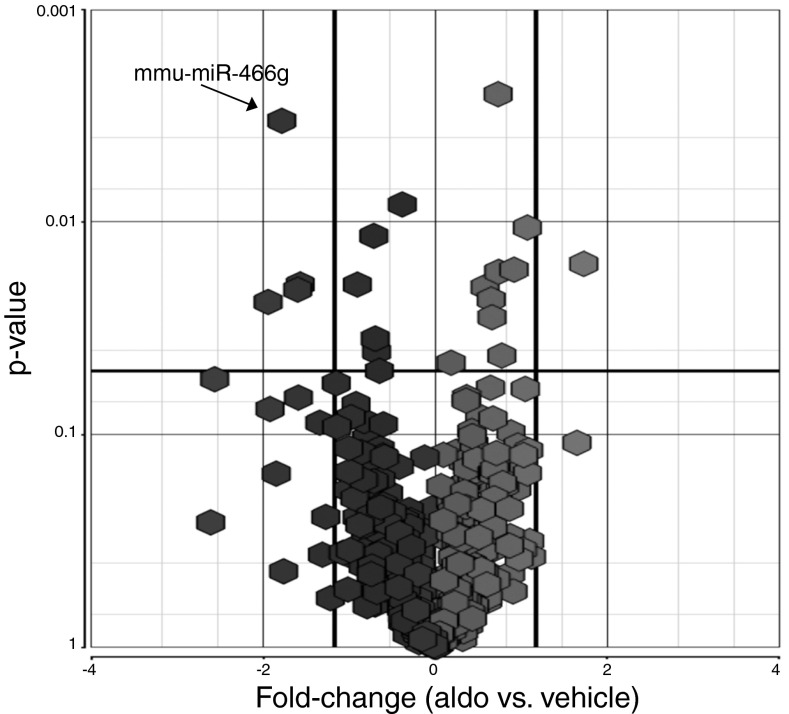
We treated polarized mpkCCDc14 cells with aldosterone (10−6 M) or ethanol vehicle for 1 h and then analyzed differential expression of miRNAs in the two groups of cells using a miRNA microarray. Out of 609 mouse miRNAs covered by the microarray, aldosterone decreased expression of 4 miRNAs and increased expression of 1 miRNA by at least 1.5-fold after 1 h of treatment (Fig. 1). The identities of these miRNAs are listed in Table 1.
Fig. 1.
Aldosterone rapidly modulates expression of a subset of miRNAs in mpkCCDc14 cells. Polarized mpkCCDc14 cells were treated with aldosterone (10−6 M) or vehicle for 1 h, and miRNAs were collected and processed for analysis by the Affymetrix GeneChip miRNA Array. This volcano map diagram shows that, of the 609 mouse miRNAs covered by the miRNA array, aldosterone decreased the expression of 4 miRNAs and increased the expression of 1 miRNA (mmu-miR-466g) by at least 1.5-fold (P < 0.05). Results are from 3 independent experiments from cells of different passages.
Table 1.
List of miRNAs differentially regulated by aldosterone in mpkCCDc14 cells
| miRNA | Fold Change (Aldosterone vs. Control) | P Value |
|---|---|---|
| mmu-miR-341 | −1.96 | 0.024 |
| mmu-miR-466 g | −1.86 | 0.003 |
| mmu-miR-466f-3p | −1.74 | 0.021 |
| mmu-miR-383 | −1.72 | 0.020 |
| mmu-miR-450a-5p | +1.82 | 0.016 |
mpkCCDc14 cells were treated with aldosterone (10−6 M) or vehicle for 1 h, and miRNAs were collected, processed, and analyzed as outlined in methods.
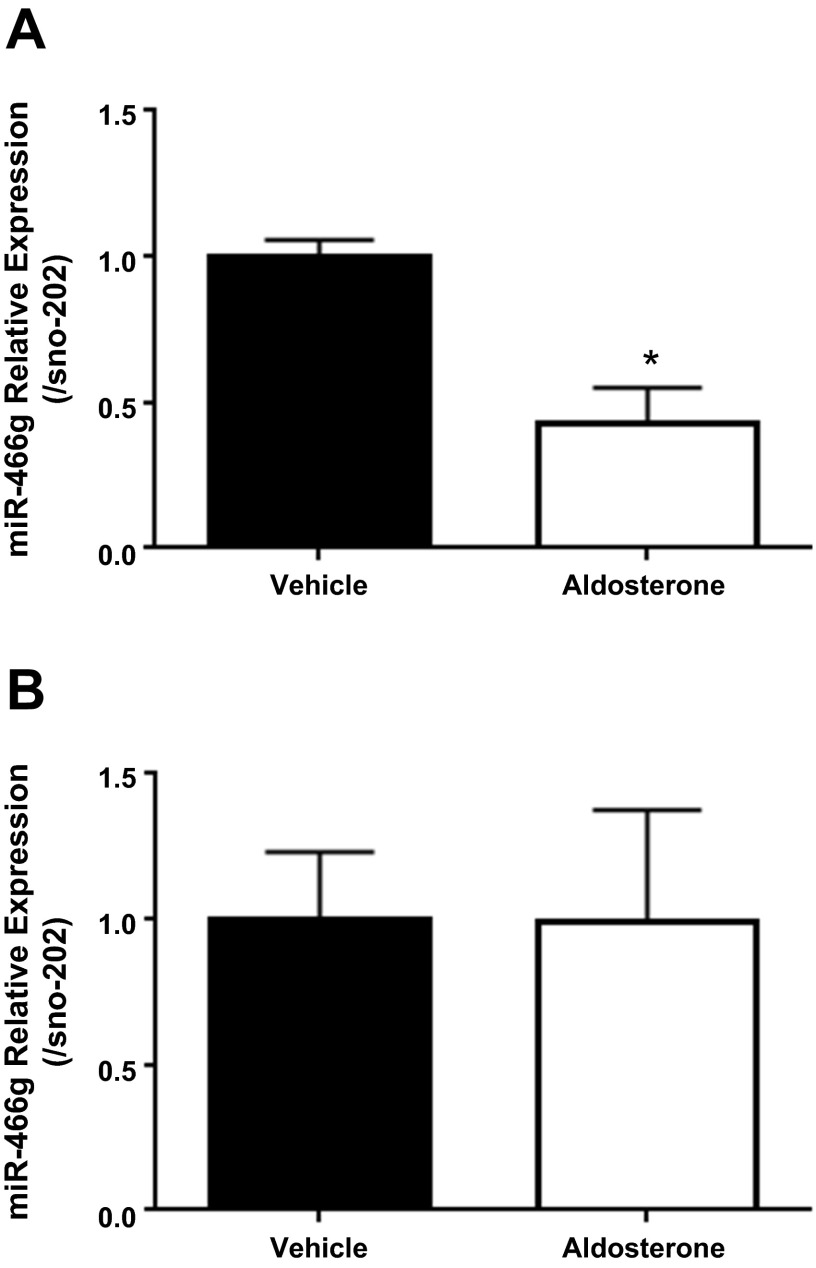
We next selected miR-466g (Ensembl Gene ID: ENSMUSG00000078025) for further characterization and used quantitative PCR to confirm that aldosterone decreases expression of miR-466g. We treated polarized mpkCCDc14 cells with aldosterone (10−6 M) or vehicle for 1 h and found that aldosterone decreased miR-466g expression by 57% (±12%) or 2.3-fold by quantitative PCR analysis (Fig. 2A). To determine whether miR-466g also qualifies as a late-phase, aldosterone-regulated miRNA, we treated mpkCCDc14 cells with aldosterone for 24 h and evaluated miR-466g expression. We found that miR-466g expression was no different between cells treated with aldosterone and vehicle for 24 h (Fig. 2B), indicating that miR-466g is strictly an early-phase aldosterone-responsive miRNA.
Fig. 2.
Aldosterone rapidly decreases expression of miR-466g in mpkCCDc14 cells. Polarized mpkCCDc14 cells were treated with aldosterone (10−6 M) or vehicle for 1 h (A) or 24 h (B), and miRNAs were collected, processed, and analyzed. Relative expression of miR-466g by quantitative PCR is shown. Expression of miR-466g is normalized to expression of sno-202. Values are means ± SE. *P < 0.05. Results are from 3 or 4 independent experiments from cells of different passages.
miR-466g interacts with the 3′-UTR of SGK1.
miRNAs select their regulatory targets by base pairing with sites in the 3′-UTR of target mRNAs. Classification of selectivity of miRNAs for target mRNAs is based on 7- to 8-mer nucleotide stretches, called seed sequences, located at the 5′-end of miRNAs, with nucleotides 2–7 of the miRNA being perfectly complementary to target mRNA sequences (5). To identify target mRNAs for miR-466g, we used online prediction programs (TargetScan, MiRanda, and PicTar) and identified a putative binding site for mmu-miR-466g in the 3′-UTR of mouse SGK1. This target sequence in the 3′-UTR of SGK1 is evolutionarily conserved throughout vertebrates (Fig. 3), suggesting that this sequence confers important properties to SGK1 expression or function.
Fig. 3.
A miR-466g target sequence is located within the 3′-UTR of SGK1. The seed sequence of miR-466g (nucleotides 2–8) perfectly matches with a sequence within nucleotides 879 to 900 (following the stop codon) of the 3′-UTR of mouse SGK1. This target sequence in the 3′-UTR of SGK1 is conserved across the indicated species (boxed area in sequence alignment).
SGK1 mRNA is an attractive candidate for miR-466g regulation because SGK1 has been established as an early-response gene whose product mediates the stimulatory effects of aldosterone on ENaC activity in CCD (17, 22, 24, 28, 33). SGK1 mRNA expression increases in CCD cells as early as 15 min after mineralocorticoid or glucocorticoid stimulation (8, 27). Since miRNAs decrease gene expression by binding to sites in the 3′-UTR of target mRNAs, aldosterone might increase SGK1 mRNA expression in part by decreasing miR-466g expression.
We hypothesized that aldosterone decreases expression of miR-466g, which, in the absence of aldosterone, binds to the 3′-UTR of SGK1 to inhibit SGK1 expression. To test functional interaction between miR-466g and SGK1, we constructed an SGK1-3′-UTR luciferase reporter (SGK1-3′-UTR WT) and cotransfected HEK-293 cells with this reporter and increasing amounts of miR-466g. We found that miR-466g decreased SGK1-3′-UTR WT luciferase activity in a dose-dependent manner (38 ± 4% at 5 nM, 44 ± 3% at 30 nM, and 54 ± 3% at 100 nM), indicating that miR-466g inhibits SGK1 3′-UTR activity in this system (Fig. 4A).
To determine whether miR-466g binds to the putative miR-466g target sequence in the 3′-UTR of SGK1, we constructed two mutants of the SGK1-3′-UTR WT luciferase reporter, each containing deletion or point mutations of the miR-466g target sequence. Deletion of the miR-466g target sequence in SGK1-3′-UTR WT (SGK1-3′-UTR DEL) attenuated miR-466g-directed suppression of luciferase activity (Fig. 4B). Introduction of point mutations that disrupt the miR-466g target sequence in SGK1-3′-UTR WT (SGK1-3′-UTR MUT, Fig. 4C) also blunted miR-466g-directed suppression of luciferase activity (Fig. 4D). These studies demonstrate that miR-466g can regulate SGK1 3′-UTR activity by interacting with the miR-466g target sequence and suggest that miR-466g controls SGK1 mRNA expression in kidney cells.
miR-466g regulates aldosterone-induced SGK1 expression and ENaC current in mpkCCDc14 cells.
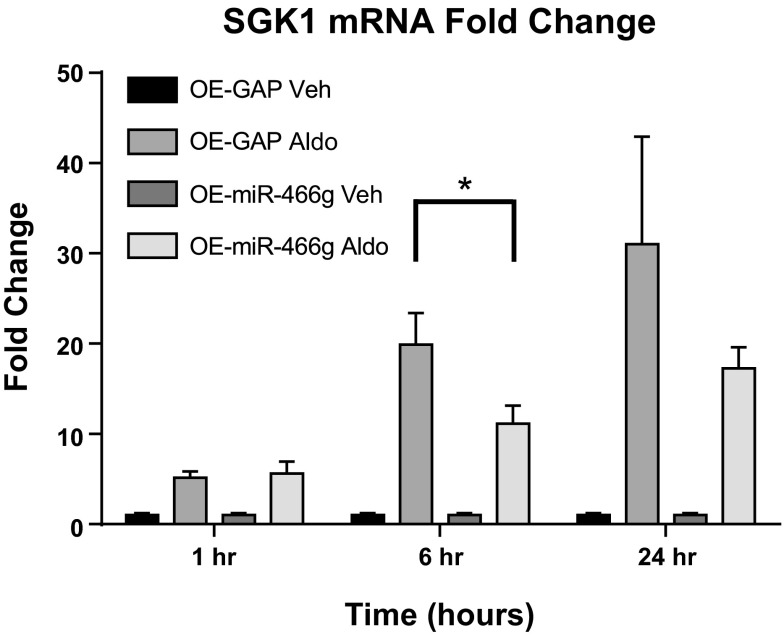
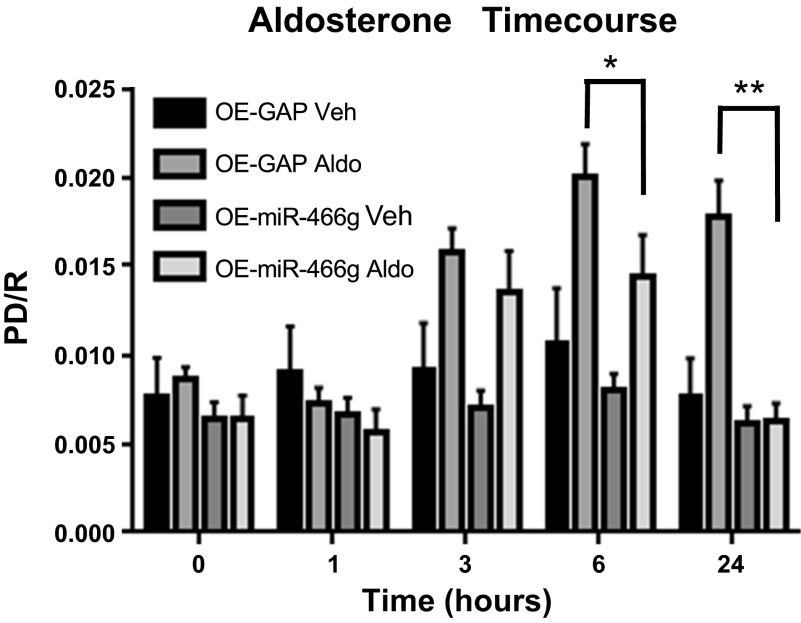
To test whether miR-466g regulates SGK1 mRNA expression in CCD cells, we stably transduced mpkCCDc14 cells to overexpress miR-466g and evaluated SGK1 mRNA expression. Stable transduction of miR-466g in mpkCCDc14 cells led to a 400-fold increase in miR-466g levels, which did not change with 1, 6, or 24 h of aldosterone induction (data not shown). We found that cells overexpressing miR-466g had 12.9-fold lower level of SGK1 mRNA compared with cells expressing control siRNA after 6 h of aldosterone induction (P < 0.05; Fig. 5). Moreover, compared with control cells, cells overexpressing miR-466g exhibited 25% decrease in amiloride-sensitive current after 6 h of aldosterone induction and complete loss of amiloride-sensitive current after 24 h of aldosterone induction (P < 0.05; Fig. 6). Together, these findings strongly suggest that miR-466g regulates aldosterone-induced SGK1 mRNA expression and ENaC current in mpkCCDc14 cells.
Fig. 5.
miR-466g decreases aldosterone-induced increase in SGK1 expression in mpkCCDc14 cells. Polarized mpkCCDc14 cells, transduced with miR-466g or nontargeting siRNA, were treated with aldosterone (10−6 M; Aldo) or vehicle (Veh) for 1, 6, or 24 h. Expression of SGK1 mRNA was measured by quantitative PCR and normalized to expression of GAPDH. Relative expression of SGK1 mRNA in cells transduced with miR-466g (OE-miR-466g) or GAPDH (OE-GAP) treated with aldosterone is shown as fold change over SGK1 mRNA in corresponding cells treated with vehicle. Values are means ± SE. *P < 0.05 between cells transduced with GAPDH and treated with aldosterone (OE-GAP Aldo) and cells transduced with miR-466g and treated with aldosterone (OE-miR-466g Aldo) at the 6-h time point. Results are from 7 independent experiments from cells of different passages.
Fig. 6.
miR-466g decreases aldosterone-induced increase in amiloride-sensitive current in mpkCCDc14 cells. Polarized mpkCCDc14 cells, transduced with miR-466g or nontargeting siRNA, were treated with aldosterone (10−6 M) or vehicle and analyzed 1, 3, 6, and 24 h after treatment. Equivalent current [potential difference/resistance (PD/R)] was measured across cell monolayers at serial time points after aldosterone treatment. Equivalent current was completely sensitive to amiloride (10−5 M) treatment. Values are means ± SE, from 12 independent experiments. *P < 0.05 between cells transduced with GAPDH and treated with aldosterone (OE-GAP Aldo) and cells transduced with miR-466g and treated with aldosterone (OE-miR-466g Aldo) at the 6-h time point. **P < 0.001 between cells transduced with GAPDH and treated with aldosterone (OE-GAP Aldo) and cells transduced with miR-466g and treated with aldosterone (OE-miR-466g Aldo) at the 24-h time point.
DISCUSSION
In the present study, we used a miRNA microarray to identify miRNAs that are acutely regulated by aldosterone in CCD cells. We identified miR-466g as a miRNA that was decreased by more than twofold within 1 h of aldosterone treatment in mpkCCDc14 cells. We also identified SGK1 as a regulatory target of miR-466g. SGK1 3′-UTR luciferase reporter assays demonstrated that miR-466g and SGK1 3′-UTR functionally interacted in kidney cells; moreover, overexpression of miR-466g in mpkCCDc14 cells decreased both aldosterone-induced SGK1 mRNA expression and amiloride-sensitive current. Together, our findings implicate miR-466g as an early-phase aldosterone-responsive miRNA that modulates SGK1 expression and ENaC activity in CCD cells.
In the early phase of induction, aldosterone activates preexisting ENaC channels through transcriptional regulation of proteins that stimulate ENaC activity (36). In particular, aldosterone stimulates expression of SGK1 in the late distal convoluted tubule, connecting segment, and collecting duct (7, 8, 18, 25, 27), which together constitute the aldosterone-sensitive distal nephron. In turn, SGK1 stimulates ENaC activity through several mechanisms, including an increase in the number and residency time of ENaC in the apical membrane (3, 10, 21, 25, 32) and an increase in open probability of ENaC (2, 11, 34, 37). SGK1 knockout mouse models have largely recapitulated the findings of cell culture models of SGK1 action; there are now three SGK1 knockout mouse models from independent groups demonstrating alterations in urinary Na+ excretion and blood pressure regulation (14, 15, 39).
SGK1 is thus an attractive candidate for miR-466g regulation because SGK1 mRNA expression changes rapidly in response to aldosterone stimulation. Several lines of evidence support our conclusion that SGK1 is a target of miR-466g regulation. First, the 3′-UTR of SGK1 contains a putative binding site for miR-466g with perfect complementarity with the seed sequence of miR-466g. Second, this site in the 3′-UTR of SGK1 is evolutionarily conserved across a wide variety of species, suggesting that this region significantly contributes to control of SGK1 expression or function. Third, cotransfection of HEK-293 cells with miR-466g and an SGK1-3′-UTR luciferase reporter decreases luciferase activity in a dose-dependent manner. Disruption of the putative miR-466g binding site in the SGK1 3′-UTR attenuates miR-466g-dependent regulation of SGK1-3′-UTR activity. Finally, overexpression of miR-466g in mpkCCDc14 cells decreases aldosterone-stimulated levels of SGK1 mRNA expression and ENaC activity.
To date, there has been one study characterizing the putative function of miR-466g in mice. Zheng and colleagues (40) identified several members of the miR-466 family that are located in the intron of Sfmbt2, a maternally imprinted polycomb gene. This miRNA cluster, which also includes the miR-290-295 families, regulates genes involved in controlling cell growth and survival in early mammalian development (40). The human counterpart of mmu-miR-466g is hsa-miR-466; the nucleotide sequences of these two miRNAs are similar in that their seed sequences are nearly identical, suggesting that hsa-miR-466 shares similar properties with mmu-miR-466g and perhaps may regulate SGK1 expression in humans. We also note that miR-466g may regulate SGK1 expression at additional sequences in the SGK1 3′-UTR because deletion or mutation of the seed sequence in the SGK1 3′-UTR does not fully prevent miR-466g-dependent regulation of SGK1-3′-UTR activity (Fig. 4).
Recent studies have begun to address the emerging role of miRNAs in regulating ion transport in the distal nephron. Elvira-Matelot et al. (13) have demonstrated that low Na+ feeding, high K+ feeding, or chronic aldosterone infusion of mice decreases renal miR-192 expression. The authors have suggested that aldosterone inhibits miR-192 expression as mechanism for increasing WNK1 (with-no-lysine kinase-1) expression in the CCD; since WNK1 regulates a number of ion transporters and channels in the distal nephron (19, 26, 35, 38), the authors propose that miR-192 partly mediates the regulatory effects of aldosterone and WNK1 on ion transport in the CCD (13). Lin et al. (23) have demonstrated that high K+ feeding of mice increases miR-802 expression in the CCD; miR-802 suppresses the expression of caveolin-1, which, in the absence of miR-802, accelerates ROMK (renal outer medullary K+) channel endocytosis. The authors propose that miR-802 mediates the stimulatory effect of high K+ diet on ROMK activity by suppressing caveolin-1 expression and increasing ROMK cell surface expression. Finally, Edinger et al. (12) have found that chronic aldosterone stimulation of mCCDc11 cells decreases expression of miR-335-3p, miR-290-5p, and miR-1983. Reducing expression of these miRNAs, individually or in combination, increases baseline ENaC activity, whereas increasing expression of these miRNAs decreases aldosterone-stimulated ENaC activity. The authors have also identified ankyrin-3 as a gene target of aldosterone and miRNA regulation, the product of which stimulates ENaC activity in mCCDc11 cells. Interestingly, there is no overlap between the set of miRNAs identified by our screen at 1 h of aldosterone stimulation of mpkCCDc14 cells and the set of miRNAs identified by Edinger and colleagues (12) at 24 h of aldosterone stimulation of mCCDc11 cells. Indeed, in our study, aldosterone decreased miR-466g expression only after 1 h, and not after 24 h, of aldosterone stimulation (Fig. 2, A and B). In aggregate, these findings suggest that distinct subsets of miRNAs mediate regulation of genes involved in early phase and late phases of aldosterone induction in kidney collecting duct cells.
Here, we expand the role of miRNAs in regulating ion transport in the distal nephron by including miR-466g as a mediator of the early effects of aldosterone on ENaC activity. Aldosterone rapidly downregulates miR-466g, which, in turn, de-represses SGK1 mRNA expression and increases ENaC activity. This represents an unrecognized mechanism for accelerating SGK1 mRNA expression in CCD cells during the early phase of aldosterone induction. When miR-466g expression is forced to be high, through stable transduction of miR-466g in mpkCCDc14 cells, SGK1 mRNA and ENaC activity decreases 6 h after aldosterone induction, suggesting that miR-466g serves as a brake for SGK1 expression and ENaC activity and that aldosterone can release this brake as a mechanism to stimulate SGK1 and ENaC activity in CCD cells. Interestingly, we found that overexpression of miR-466g diminishes aldosterone-induced ENaC activity to a greater extent than it diminishes aldosterone-induced SGK1 mRNA expression. This discrepancy may reflect additional unidentified gene targets of miR-466g that can regulate ENaC activity in mpkCCDc14 cells. Future studies will be necessary to identify these putative gene targets and to determine whether aberrant regulation of miR-466g expression in the CCD leads to abnormalities in renal Na+ handling and blood pressure regulation in vivo.
In conclusion, we have identified miR-466g as a miRNA that is rapidly decreased by aldosterone stimulation in CCD cells. In turn, miR-466g regulates SGK1 mRNA expression and ENaC activity in CCD cells. We propose that, at baseline, miR-466g suppresses SGK1 mRNA expression in CCD cells; with aldosterone stimulation, miR-466g expression rapidly decreases, which serves to accelerate SGK1 mRNA expression. These findings suggest a novel mechanism by which aldosterone rapidly increases ENaC activity in CCD cells.
GRANTS
This work was supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (T32-DK07357-26A1 to P. P. Kathpalia and M. E. Jacobs; and K08-DK073487 to A. C. Pao), Satellite Healthcare (2008 Norman S. Coplon Extramural Grant to A. C. Pao), and the Palo Alto Institute for Research and Education (seed grant to A. C. Pao). This work was also supported by the Mervin G. Morris Research Fund in the Division of Nephrology at Stanford University.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
M.E.J., P.P.K., Y.C., and S.V.T. performed experiments; M.E.J., P.P.K., Y.C., S.V.T., E.J.N., and A.C.P. analyzed data; M.E.J., P.P.K., E.J.N., and A.C.P. interpreted results of experiments; M.E.J., P.P.K., Y.C., S.V.T., and A.C.P. prepared figures; M.E.J., P.P.K., and A.C.P. drafted manuscript; M.E.J., P.P.K., and A.C.P. edited and revised manuscript; M.E.J., P.P.K., and A.C.P. approved final version of manuscript; P.P.K., E.J.N., and A.C.P. conception and design of research.
ACKNOWLEDGMENTS
We are grateful to Dr. Alain Vandewalle (Institut National de la Santé et de la Recherche Médicale) for providing mpkCCDc14 cells for our experiments. We thank Drs. Vivek Bhalla (Stanford University), Glenn Chertow (Stanford University), and Michael Butterworth (University of Pittsburgh) for valuable discussions.
REFERENCES
- 1.Alvarez de la Rosa D, Canessa CM. Role of SGK in hormonal regulation of epithelial sodium channel in A6 cells. Am J Physiol Cell Physiol 284: C404–C414, 2003. [DOI] [PubMed] [Google Scholar]
- 2.Alvarez de la Rosa D, Paunescu TG, Els WJ, Helman SI, Canessa CM. Mechanisms of regulation of epithelial sodium channel by SGK1 in A6 cells. J Gen Physiol 124: 395–407, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alvarez de la Rosa D, Zhang P, Naray-Fejes-Toth A, Fejes-Toth G, Canessa CM. The serum and glucocorticoid kinase sgk increases the abundance of epithelial sodium channels in the plasma membrane of Xenopus oocytes. J Biol Chem 274: 37834–37839, 1999. [DOI] [PubMed] [Google Scholar]
- 4.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, function. Cell 116: 281–297, 2004. [DOI] [PubMed] [Google Scholar]
- 5.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell 136: 215–233, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bens M, Vallet V, Cluzeaud F, Pascual-Letallec L, Kahn A, Rafestin-Oblin ME, Rossier BC, Vandewalle A. Corticosteroid-dependent sodium transport in a novel immortalized mouse collecting duct principal cell line. J Am Soc Nephrol 10: 923–934, 1999. [DOI] [PubMed] [Google Scholar]
- 7.Bhargava A, Fullerton MJ, Myles K, Purdy TM, Funder JW, Pearce D, Cole TJ. The serum- and glucocorticoid-induced kinase is a physiological mediator of aldosterone action. Endocrinology 142: 1587–1594, 2001. [DOI] [PubMed] [Google Scholar]
- 8.Chen SY, Bhargava A, Mastroberardino L, Meijer OC, Wang J, Buse P, Firestone GL, Verrey F, Pearce D. Epithelial sodium channel regulated by aldosterone-induced protein sgk. Proc Natl Acad Sci U S A 96: 2514–2519, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dai R, Phillips RA, Zhang Y, Khan D, Crasta O, Ahmed SA. Suppression of LPS-induced Interferon-gamma and nitric oxide in splenic lymphocytes by select estrogen-regulated microRNAs: a novel mechanism of immune modulation. Blood 112: 4591–4597, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Debonneville C, Flores SY, Kamynina E, Plant PJ, Tauxe C, Thomas MA, Munster C, Chraibi A, Pratt JH, Horisberger JD, Pearce D, Loffing J, Staub O. Phosphorylation of Nedd4-2 by Sgk1 regulates epithelial Na(+) channel cell surface expression. EMBO J 20: 7052–7059, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diakov A, Korbmacher C. A novel pathway of epithelial sodium channel activation involves a serum- and glucocorticoid-inducible kinase consensus motif in the C terminus of the channel's alpha-subunit. J Biol Chem 279: 38134–38142, 2004. [DOI] [PubMed] [Google Scholar]
- 12.Edinger RS, Coronnello C, Bodnar AJ, LaFramboise WA, Benos PV, Ho J, Johnson JP, Butterworth MB, Bhalla V, Labarca M. Aldosterone regulates microRNAs in the cortical collecting duct to alter sodium transport. J Am Soc Nephrol 25: 2445–2457, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elvira-Matelot E, Zhou XO, Farman N, Beaurain G, Henrion-Caude A, Hadchouel J, Jeunemaitre X. Regulation of WNK1 expression by miR-192 and aldosterone. J Am Soc Nephrol 21: 1724–1731, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Faresse N, Lagnaz D, Debonneville A, Ismailji A, Maillard M, Fejes-Toth G, Naray-Fejes-Toth A, Staub O. Inducible kidney-specific Sgk1 knockout mice show a salt-losing phenotype. Am J Physiol Renal Physiol 302: F977–F985, 2012. [DOI] [PubMed] [Google Scholar]
- 15.Fejes-Toth G, Frindt G, Naray-Fejes-Toth A, Palmer LG. Epithelial Na+ channel activation and processing in mice lacking SGK1. Am J Physiol Renal Physiol 294: F1298–F1305, 2008. [DOI] [PubMed] [Google Scholar]
- 16.Fiedler SD, Carletti MZ, Hong X, Christenson LK. Hormonal regulation of microRNA expression in periovulatory mouse mural granulosa cells. Biol Reprod 79: 1030–1037, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Helms MN, Fejes-Toth G, Naray-Fejes-Toth A. Hormone-regulated transepithelial Na+ transport in mammalian CCD cells requires SGK1 expression. Am J Physiol Renal Physiol 284: F480–F487, 2003. [DOI] [PubMed] [Google Scholar]
- 18.Hou J, Speirs HJ, Seckl JR, Brown RW. Sgk1 gene expression in kidney and its regulation by aldosterone: spatio-temporal heterogeneity and quantitative analysis. J Am Soc Nephrol 13: 1190–1198, 2002. [PubMed] [Google Scholar]
- 19.Huang CL, Yang SS, Lin SH. Mechanism of regulation of renal ion transport by WNK kinases. Curr Opin Nephrol Hypertens 17: 519–525, 2008. [DOI] [PubMed] [Google Scholar]
- 20.Jacobs ME, Jeffers LA, Welch AK, Wingo CS, Cain BD. MicroRNA regulation of endothelin-1 mRNA in renal collecting duct cells. Life Sci 118: 195–199, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Knight KK, Olson DR, Zhou R, Snyder PM. Liddle's syndrome mutations increase Na+ transport through dual effects on epithelial Na+ channel surface expression and proteolytic cleavage. Proc Natl Acad Sci U S A 103: 2805–2808, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lang F, Bohmer C, Palmada M, Seebohm G, Strutz-Seebohm N, Vallon V. (Patho)physiological significance of the serum- and glucocorticoid-inducible kinase isoforms. Physiol Rev 86: 1151–1178, 2006. [DOI] [PubMed] [Google Scholar]
- 23.Lin DH, Yue P, Pan C, Sun P, Wang WH. MicroRNA 802 stimulates ROMK channels by suppressing caveolin-1. J Am Soc Nephrol 22: 1087–1098, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Loffing J, Flores SY, Staub O. Sgk kinases and their role in epithelial transport. Annu Rev Physiol 68: 461–490, 2006. [DOI] [PubMed] [Google Scholar]
- 25.Loffing J, Zecevic M, Feraille E, Kaissling B, Asher C, Rossier BC, Firestone GL, Pearce D, Verrey F. Aldosterone induces rapid apical translocation of ENaC in early portion of renal collecting system: possible role of SGK. Am J Physiol Renal Physiol 280: F675–F682, 2001. [DOI] [PubMed] [Google Scholar]
- 26.McCormick JA, Ellison DH. The WNKs: atypical protein kinases with pleiotropic actions. Physiol Rev 91: 177–219, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Naray-Fejes-Toth A, Canessa C, Cleaveland ES, Aldrich G, Fejes-Toth G. SGK is an aldosterone-induced kinase in the renal collecting duct Effects on epithelial Na+ channels. J Biol Chem 274: 16973–16978, 1999. [DOI] [PubMed] [Google Scholar]
- 28.Pao AC. SGK regulation of renal sodium transport. Curr Opin Nephrol Hypertens 21: 534–540, 2012. [DOI] [PubMed] [Google Scholar]
- 29.Pao AC, Bhargava A, Di Sole F, Quigley R, Shao X, Wang J, Thomas S, Zhang J, Shi M, Funder JW, Moe OW, Pearce D. Expression and role of serum and glucocorticoid-regulated kinase 2 in the regulation of Na+/H+ exchanger 3 in the mammalian kidney. Am J Physiol Renal Physiol 299: F1496–F1506, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rougvie AE. miRNA regulation through ligand occupancy of a nuclear hormone receptor. Sci Signal 2: pe52, 2009. [DOI] [PubMed] [Google Scholar]
- 31.Shi XB, Xue L, Yang J, Ma AH, Zhao J, Xu M, Tepper CG, Evans CP, Kung HJ, deVere White RW. An androgen-regulated miRNA suppresses Bak1 expression and induces androgen-independent growth of prostate cancer cells. Proc Natl Acad Sci U S A 104: 19983–19988, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Snyder PM, Olson DR, Thomas BC. Serum and glucocorticoid-regulated kinase modulates Nedd4-2-mediated inhibition of the epithelial Na+ channel. J Biol Chem 277: 5–8, 2002. [DOI] [PubMed] [Google Scholar]
- 33.Soundararajan R, Pearce D, Hughey RP, Kleyman TR. Role of epithelial sodium channels and their regulators in hypertension. J Biol Chem 285: 30363–30369, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thomas SV, Kathpalia PP, Rajagopal M, Charlton C, Zhang J, Eaton DC, Helms MN, Pao AC. Epithelial sodium channel regulation by cell surface-associated serum- and glucocorticoid-regulated kinase 1. J Biol Chem 286: 32074–32085, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Uchida S. Pathophysiological roles of WNK kinases in the kidney. Pflügers Arch 460: 695–702, 2010. [DOI] [PubMed] [Google Scholar]
- 36.Verrey F, Pearce D, Pfeiffer R, Spindler B, Mastroberardino L, Summa V, Zecevic M. Pleiotropic action of aldosterone in epithelia mediated by transcription and post-transcription mechanisms. Kidney Int 57: 1277–1282, 2000. [DOI] [PubMed] [Google Scholar]
- 37.Vuagniaux G, Vallet V, Jaeger NF, Hummler E, Rossier BC. Synergistic activation of ENaC by three membrane-bound channel-activating serine proteases (mCAP1, mCAP2, and mCAP3) and serum- and glucocorticoid-regulated kinase (Sgk1) in Xenopus Oocytes. J Gen Physiol 120: 191–201, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Welling PA, Chang YP, Delpire E, Wade JB. Multigene kinase network, kidney transport, and salt in essential hypertension. Kidney Int 77: 1063–1069, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wulff P, Vallon V, Huang DY, Volkl H, Yu F, Richter K, Jansen M, Schlunz M, Klingel K, Loffing J, Kauselmann G, Bosl MR, Lang F, Kuhl D. Impaired renal Na(+) retention in the sgk1-knockout mouse. J Clin Invest 110: 1263–1268, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zheng GX, Ravi A, Gould GM, Burge CB, Sharp PA. Genome-wide impact of a recently expanded microRNA cluster in mouse. Proc Natl Acad Sci U S A 108: 15804–15809, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]