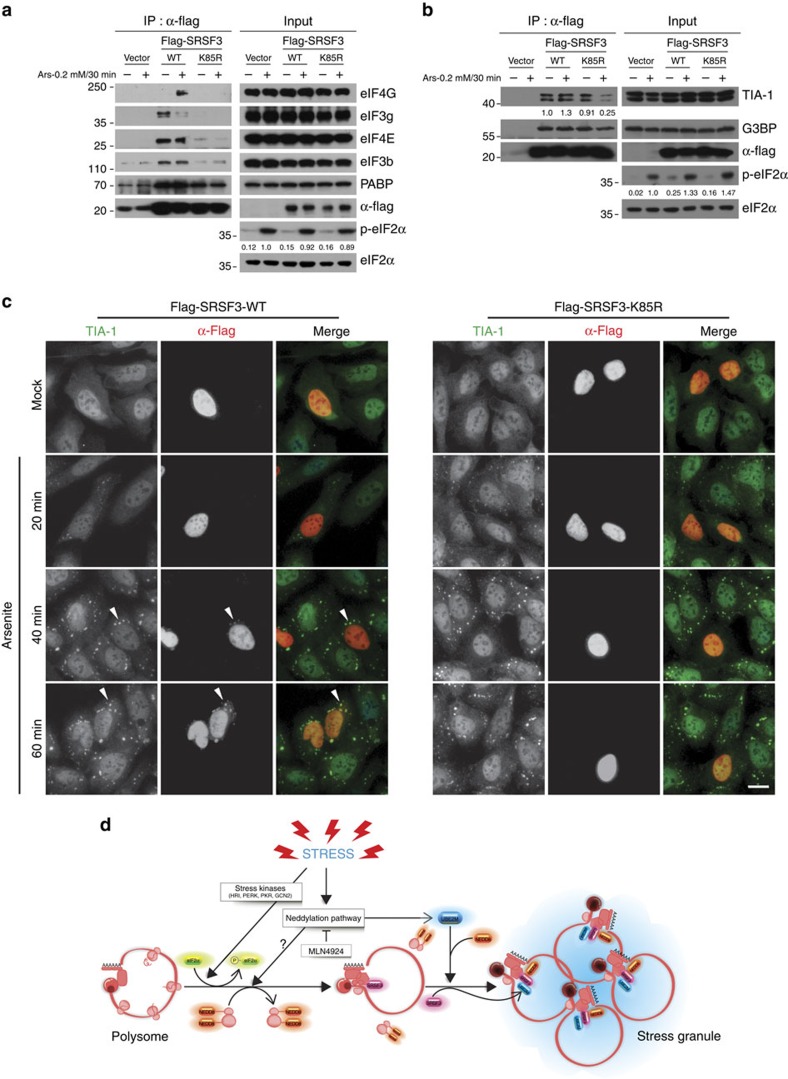
Figure 7. SRSF3–K85R displays defects in association with SG components.
(a) Empty vector, Flag–SRSF3-WT or K85R mutant transfected cells were treated with arsenite for 30 min, lysed in IP buffer and immunoprecipitated using Flag agarose beads. The precipitates were subjected to western analysis using antibodies against SG proteins. (b) SRSF3–K85R has a defect in associating with TIA-1 under arsenite stress. Immunoprecipitation was carried out as described in a and the precipitates were blotted against TIA-1 and G3BP. (c) U2OS cells transiently transfected with Flag–SRSF3-WT or K85R mutant were treated with either mock or 0.2 mM arsenite and subjected to immunofluorescence microscopy. Recruitment of TIA-1 into SGs was assessed by co-staining with anti-TIA-1 and anti-Flag antibodies. Arrowhead indicates SG. (d) Model diagram of SG assembly mediated by neddylation pathway. During stressful condition, stress kinases (HRI, PKR, PERK, and GCN2) are activated and phosphorylate eIF2α to initiate abortive translation initiation and polysome disassembly. Subsequently, neddylation pathway possibly targets ribosomal proteins to promote polysome disassembly. Finally, neddylation of SRSF3 promotes SG aggregation through interaction with initiation factors such as eIF4G, eIF3s and SG assembly factor TIA-1. Scale bar, 10 μm.