Abstract
Nuclear protein of the testis (NUT) midline carcinomas are rare aggressive carcinomas characterized by chromosomal rearrangements that involve the gene encoding the NUT. This article reviews the clinicopathologic features and the differential diagnosis of these malignancies.
Key words: Nuclear protein of the testis midline carcinoma, primary mediastinal seminoma, histone deacetylase inhibitors
Introduction
Germ cell tumors are classified as extragonadal, if there is no evidence of a primary tumor in the testis or ovaries.1 Primary extragonadal germ cell tumors (EGGCT) constitute a small subset of 2-5% of all germ cell tumors; and primary mediastinal seminoma (PMS) include only 10% of EGGCT.2 These tumors occur almost exclusively in males, and the age at presentation is generally 20-35 years.3 More than a third of all malignant germ cell tumors are pure seminomas.3 Seminoma is one of the most radio and chemo sensitive tumors.4 When treated, the prognosis is good, with a mean survival of 90% at five years.5 Aggressive variant of PMS are rarely described in the literature.1-5
NUT midline carcinomas (NMCs) are rare aggressive carcinomas characterized by chromosomal rearrangements that involve the gene encoding the nuclear protein of the testis (NUT).6 NMC is named for its tendency to involve midline structures.7 The most common tumor localization at the time of diagnosis are the head and neck region and mediastinum. To date, at least 63 cases have been reported. It affects people of all ages (range 0-78 years), though a skew towards younger individuals may represent selection bias.8 The histological diagnosis is usually that of poorly differentiated or squamous cell carcinoma but occasionally has been classified as other tumors, e.g. seminoma.9 Even with multimodality therapy the median survival from diagnosis is only 6.7 months.8 Herein we describe a case of an aggressive form of disease and discuss the differential diagnosis of this lesion.
Case Report
In February 2013, a 28-year-old male was presented with a history of progressive dyspnea, cough, hoarseness of voice, and unquantified weight loss during the last 3 months. Chest x-ray showed mediastinal widening and chest computed tomography (CT) imaging revealed a large mediastinal mass measuring 15.3×8.4×14.7 cm (Figure 1). Additionally, extensive mediastinal and left hilar lymphadenopathy was seen on chest CT. Clinical suspicion of lymphoma led to bone marrow biopsy. The laboratory report ruled out lymphoma. A thoracoscopic mediastinal biopsy was performed. This showed a poorly differentiated carcinoma most likely consistent with seminoma (histological features to be described). The patient was admitted to N.N. Blokhin Russian Cancer Research Center for further work-up and evaluation.
Figure 1.
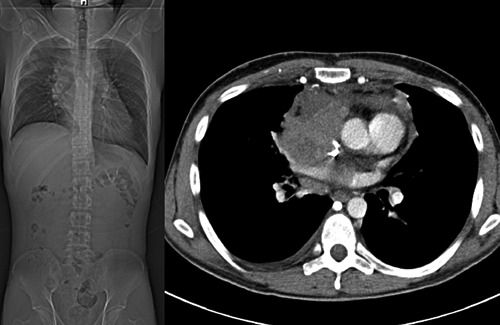
Coronal computed tomography image of the chest and abdomen before treatment.
At admission, there were signs of superior vena cava syndrome. The serum lactate dehydrogenase (LDH) levels were raised to 840 U/L (normal range <450 U/L) while b- human chorionic gonadotropin (b-HCG<0.12 mlU/mL) and alpha fetoprotein (AFP 1.26 ng/mL) levels were normal. Considering the tumor morphology, the young age, clinical presentation, it was decided to treat the patient as extragonadal seminoma. The patient received chemotherapy comprising of bleomycin, etoposide, and cisplatin (BEP) 3 weekly for three cycles until August 2013. The patient tolerated chemotherapy well with no dose reductions. There was a serial reduction in serum LDH levels. The patient achieved a partial response. Neither surgery nor radiation therapy was performed because of the size and location of the lesion. Two months after the completion of chemotherapy, the patient presented with marked dyspnea at rest. Positron emission tomography-CT (PET-CT) confirmed tumor recurrence with raised serum LDH levels (1126 U/L). The serum b-HCG and AFP levels were normal. The patient was treated with paclitaxel, ifosfamide and cisplatin (TIP) until December 2013. A chest CT scan following three cycles of TIP regimen showed a mediastinal mass that had significantly increased in size, infiltrating superior vena cava and right atrium. Liver metastases were also detected (cytological findings – most likely seminoma). Salvage chemotherapy with gemcitabine and oxaliplatin (GEMOX) was started. The patient received only two cycles before developing acute pulmonary embolism. The CT showed increase in size of liver metastases with raised serum LDH levels (2170 U/L). Given the non-standard clinical course of the disease (progression after three lines of chemotherapy), it was decided to perform a biopsy of cervical-supraclavicular lymph node.
Histologic findings from the cervical-supraclavicular lymph node
The tumor had mixed undifferentiated small cells with foci of squamous differentiation. The tumor cells demonstrated immunoreactivity for pan-cytokeratin, cytokeratin 18, CD117, cytokeratin 5, p63, whereas synaptophisin, CK7, TTf1, PLAP, Oct-3/4, CD5, CD34 and CD56 were negative. The diagnosis of NUT midline carcinoma (NMC) was made after demonstration of diffuse nuclear immunohistochemical staining with NUT protein using anti-NUT rabbit polyclonal antibody (clone C52, 1:100) (Figure 2).
Figure 2.
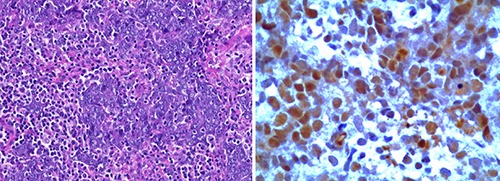
Diagnosis of nuclear protein of the testis (NUT) midline carcinoma. A) Undifferentiated small cells with foci of squamous differentiation (Hematoxylin & Eosin, 20×). B) Immunohistochemistry of tumor cell nuclei showing speckled staining for NUT (250×) using anti-NUT rabbit polyclonal antibody (clone C52, 1:100).
External consultation was pursued from the International NUT Midline Carcinoma Registry at the Brigham and Women’s Hospital. The optimal treatment for patients with NMC is unclear. Efforts have been made to develop targeted therapeutic approaches such as histone deacetylase inhibitors (HDACi). HDACi demonstrated promising clinical response in pediatric patients.10 Considering these data, treatment with quisinostat (HDACi) was initiated in February 2014. Following quisinostat treatment, Chest CT scan showed a mediastinal mass with involvement of major vessels, right atrium and right ventricle, metastases in liver that had significantly increased in 1 month (Figure 3). In conjunction with marked increase in serum LDH levels (6881U/L) it was considered as disease progression. Following disease progression, the patient received palliative treatment and died soon afterward.
Figure 3.
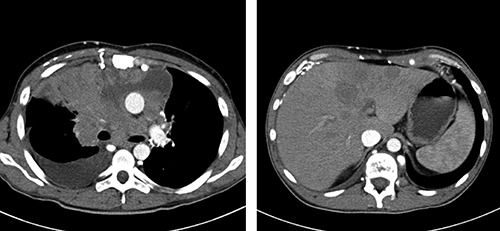
A) Coronal computed tomography (CT) image of the chest after treatment with histone deacetylase inhibitors, indicating marked adverse change from the time of diagnosis. There is encasement of major vessels, right atrium and right ventricle. B) Coronal CT image of the abdomen. There are metastases in liver that had significantly increased in size.
Discussion
NMC is a rare aggressive cancer of squamous cell lineage arising in midline structures.9 NMC are not classified according to the type of tissue and location of tumor origin in majority of solid tumors, but it’s possible to define them genetically. The specific cytogenetic abnormality is a reciprocal translocation of the NUT (nuclear protein in testis, AKA Chr15orf55) gene on the long arm of chromosome 15 with one of the BET family members, most commonly BRD4 (also known as MCAP and HUNK1) on chromosome 19p13.1 [t(15;19)(q14;p13.1)].8 The translocation results into a fusion oncogene (BRD4-NUT) which arrests normal cellular differentiation. In the remaining cases, it is either fused to BRD3 [t(9;15)(q34.2;q14), BRD3-NUT], or it is fused to uncharacterized gene(s) (NUT-variant).11 In all of previously described variants, both NUT and BET genes are fused. Moreover it was described a novel fusion gene in NMC that does not include a BET protein, but rather a BET-binding protein, NSD3.12 NSD3 is a histone methyl-transferase that belongs to the mammalian Nuclear SET Domain-containing (NSD) protein family of SET domain-containing methyltransferases. NSD3-NUT fusion oncogene encodes a protein that is both necessary and sufficient for the blockade of differentiation in NMC.12 The NUT translocation can be diagnosed using karyotype, FISH or RT-PCR. A monoclonal antibody has been developed for immunohistochemistry.13 The immunohistochemical assay can detect the NUT protein in NMC, whose expression in normal mature adult tissue is restricted to the testis.14
BRD4-NUT causes malignancy by blocking the differentiation of NMC cells and maintaining their proliferation, as evidenced by the rapid squamous differentiation that occurs following siRNA knockdown of the BRD4-NUT oncoprotein.15 The NUT portion of BRD4-NUT binds to and activates a histone acetyl-transferase, p300, which is hypothesized to acetylate regional chromatin, recruiting more local BRD4-NUT, and more local p300, leading to more local acetylation, in a self-perpetuating process that leads to the BRD4-NUT foci that can be seen by immunohistochemistry.16 The paradox is that these large aggregates of hyperacetylated chromatin, BRD4-NUT, and p300, rather than leading to increased transcription, actually act as p300 sinks that globally decrease acetylation and transcription. Thus, genes required for differentiation are not expressed. Two therapies, which target this mechanism have emerged, including bromodomain inhibitors (BETi) and HDACi, both of which induce differentiation and growth arrest of NMC cells, both in vitro and in vivo. BETi is available to adults with NMC and other solid tumors through a clinical trial.7 Histone modifying enzymes, including the NSD family, are often deregulated in cancer and aberrant histone modification profiles are intimately linked to carcinogenesis.17 The findings have led to the recent treatment of pediatric patients with NMC using HDACi-containing regimens. Results have been promising.7,10,16 But in our case we describe that, HDACi did not satisfy expectations. The patient did not respond to HDACi treatment. Others inhibitors to histone methyltransferases, including the NSD family, are under development, and this approach still holds promise for NUT midline carcinoma as well as other cancers.17,18 NMC is often widely metastatic and unresectable when diagnosed. All known cases of NMC have had a poor clinical course with a mean survival of approximately seven months.8 The differential diagnosis of NMCs, particularly when dealing with small biopsies, can be quite broad ranging from hematolymphoid malignancies, melanomas, Ewing sarcoma/primitive neuroectodermal tumors, rhabdomyosarcoma, olfactory neuroblastoma, and germ cell tumors to undifferentiated carcinomas.15,19 NMC should be considered in the differential diagnosis of any poorly differentiated epithelioid mediastinal tumor, regardless of age.20 As in our case these tumors are virtually refractory to chemotherapy. However, the knowledge about the underlying molecular alterations can serve as guide towards developing an effective treatment for this disease.9
References
- 1.Droz JP, Horwich A. Extragonadal germ cell tumors. Vogelzang NJ, Scardino PT, Shipley WU, Coffey DS. (eds). Comprehensive textbook of genitourinary oncology. 2nd ed. Philadelphia: Lippincott Williams and Wilkins; 2000. [Google Scholar]
- 2.Bokemeyer C, Nichols CR, Droz JP, et al. Extragonadal germ cell tumors of the mediastinum and retroperitoneum: results from an international analysis. J Clin Oncol 2002;20:1864-73. [DOI] [PubMed] [Google Scholar]
- 3.Chaganti RS, Houldsworth J. Genetics and biology of adult human male germ cell tumors. Cancer Res 2000;60:1475-82. [PubMed] [Google Scholar]
- 4.Nachankar A, Krishnatry R, Joshi A, et al. Primary mediastinal seminoma; resistance and relapse: an aggressive entity. Indian J Med Paediatr Oncol 2013; 34:309-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gândara F, Leitao A, Bernardo M, Ceia F. Mediastinal seminoma: a case report. Internet J Intern Med 2011;9:1. [Google Scholar]
- 6.Edward B, Stelow A. Review of NUT midline carcinoma. Head Neck Pathol 2011;5:31-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.French CA. The importance of diagnosing NUT midline carcinoma. Head Neck Pathol 2013;7:11-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bauer DE, Mitchell CM, Strait KM, et al. Clinicopathologic features and long-term outcomes of NUT midline carcinoma. Clin Cancer Res 2012;18:5773-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ball A, Bromley A, Glaze S, et al. A rare case of NUT midline carcinoma. Gynecol Oncol Case Rep 2013;3:1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schwartz BE, Hofer MD, Lemieux ME, et al. Differentiation of NUT midline carcinoma by epigenomic reprogramming. Cancer Res 2011;71:2686-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French CA, Ramirez CL, Kolmakova J, et al. BRD-NUT oncoproteins: a family of closely related nuclear proteins that block epithelial differentiation andmaintain the growth of carcinoma cells. Oncogene 2008;27:2237-42. [DOI] [PubMed] [Google Scholar]
- 12.French CA, Rahman S, Walsh EM, et al. NSD3-NUT fusion oncoprotein in NUT midline carcinoma: implications for a novel oncogenic mechanism. Cancer Discov 2014;4:928-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haack H, Johnson LA, Fry CJ, et al. Diagnosis of NUT midline carcinoma using a NUT-specific monoclonal antibody. Am J Surg Pathol 2009;33:984-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.French CA. Pathogenesis of NUT midline carcinoma. Annu Rev Pathol 2012;7:247-65. [DOI] [PubMed] [Google Scholar]
- 15.French CA, Kutok JL, Faquin WC, et al. Midline carcinoma of children and young adults with NUT rearrangement. J Clin Oncol 2004;22:4135-9. [DOI] [PubMed] [Google Scholar]
- 16.Reynoird N, Schwartz BE, Delvecchio M, et al. Oncogenesis by sequestration of CBP/p300 in transcriptionally inactive hyperacetylated chromatin domains. EMBO J 2010;29:2943-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bannister AJ, Kouzarides T. Regulation of chromatin by histone modifications. Cell Res 2011;21:381-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morishita M, di Luccio E. Cancers and the NSD family of histone lysine methyltransferases. Biochim Biophys Acta 2011;1816: 158-63. [DOI] [PubMed] [Google Scholar]
- 19.Stelow EB, French CA. Carcinomas of the upper aerodigestive tract with rearrangement of the nuclear protein of the testis (NUT) gene (NUT midline carcinomas). Adv Anat Pathol 2009;16:92-6. [DOI] [PubMed] [Google Scholar]
- 20.Evans AG, French CA, Cameron MJ, et al. Pathologic characteristics of NUT midline carcinoma arising in the mediastinum. Am J Surg Pathol 2012;36:1222-7. [DOI] [PMC free article] [PubMed] [Google Scholar]


