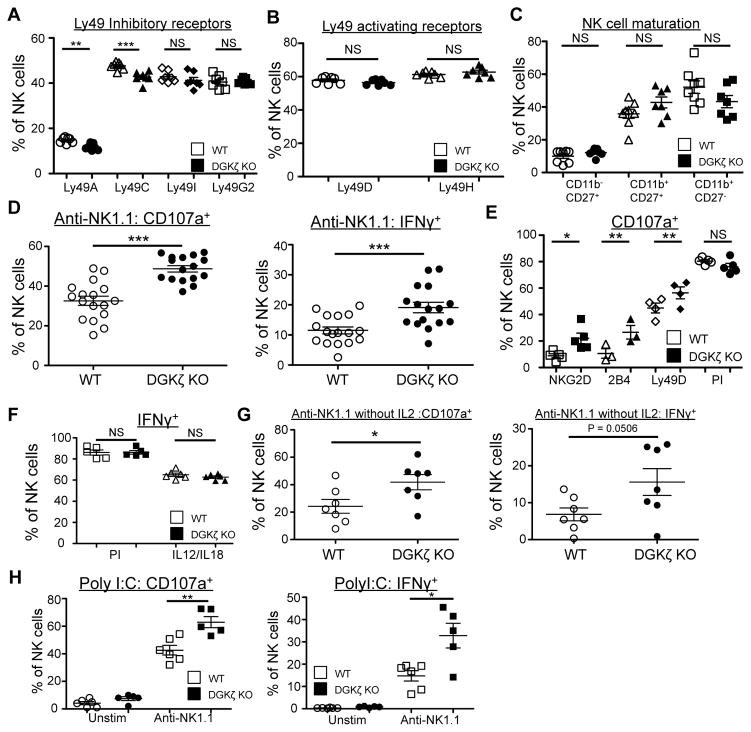
Figure 1. DGKζ-deficient NK cells exhibit enhanced function downstream of activating receptors.
The proportion of NK (CD3− NK1.1+) cells expressing A) inhibitory and B) activating Ly49 receptors, and C) CD27/CD11b in WT and DGKζ KO cells is shown. N=7. D) Splenocytes from WT and DGKζ KO mice were stimulated with plate-bound anti-NK1.1 antibody. The proportion of NK cells (CD3−DX5+NKp46+) labeled with anti-CD107a (left) and intracellular anti-IFNγ antibody (right) is shown. N=17. E) Splenocytes from WT and DGKζ KO mice were stimulated with plate-bound antibodies against the indicated activating receptors or with PMA/Ionomycin (PI). The proportion of NK cells (CD3−DX5+NKp46+) labeled with anti-CD107a antibody is shown. N=4–5 per condition. F) Splenocytes from WT and DGKζ KO mice were stimulated with PMA/ionomycin or with IL-12 and IL-18. The proportion of NK cells (CD3−DX5+NKp46+) expressing intracellular anti-IFNγ is shown. N=5–6 per condition G) Splenocytes from WT and DGKζ KO mice were stimulated with plate-bound anti-NK1.1 antibody without IL-2. The proportion of NK cells (CD3−DX5+NKp46+) labeled with anti-CD107a (left) and intracellular anti-IFNγ antibody (right) is shown. N=7. H) Splenocytes from polyI:C-treated WT and DGKζ KO mice were stimulated with plate-bound anti-NK1.1 antibodies. The proportion of NK cells (CD3−DX5+NKp46+) labeled with anti-CD107a (left) and intracellular anti-IFNγ antibody (right) is shown. N=5. *, ** and *** represent statistical significance of p<0.05, p<0.01, and p<0.001 by Student’s t-test, respectively. NS = not significant. Data shown are compiled from 2 separate experiments are shown in A–C and E, and from at least 3 separate experiments for figures D, F and G.