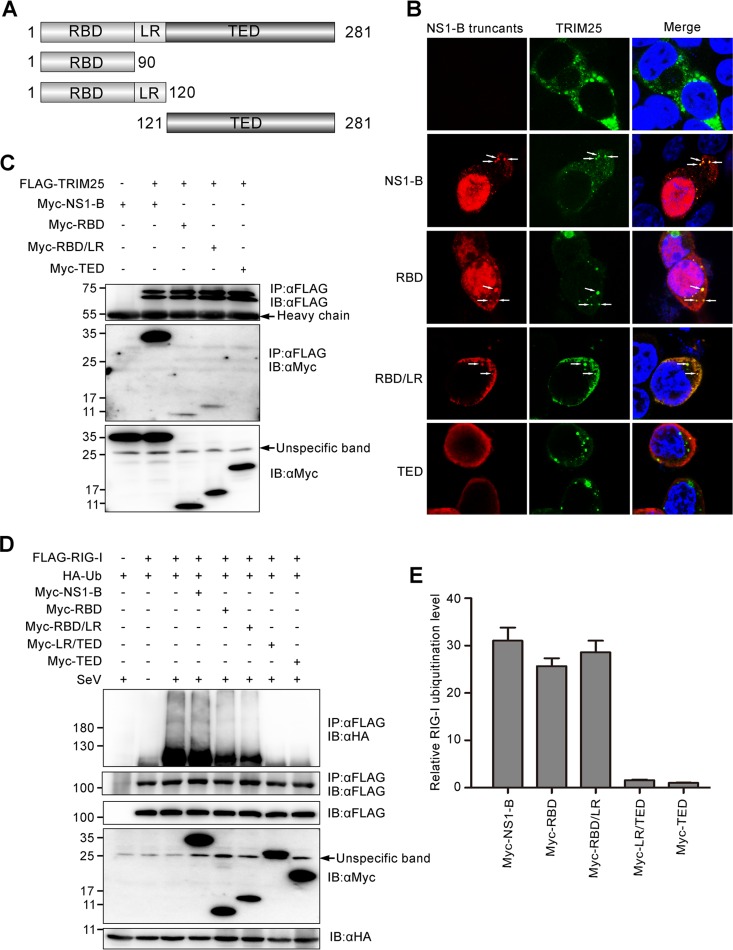
FIG 6.
The N terminus of NS1-B is responsible for interaction with TRIM25 in the RIG-I/TRIM25/NS1-B ternary complex, and this interaction negates the inhibitory ability of the C terminus of NS1-B on RIG-I ubiquitination. (A) Schematic representation of the constructs encoding Myc fused to the full-length NS1-B or NS1-B truncations. (B) Colocalization of TRIM25 and NS1-B or NS1-B truncations, as assessed by fluorescence microscopy. FLAG-TRIM25 and Myc-NS1-B or Myc-NS1-B truncations were cotransfected into 293T cells for 24 h, followed by staining with anti-FLAG (green) and anti-Myc (red) antibodies. The nucleus was stained with DAPI (blue). White arrows indicate the granule-like aggregates. (C) FLAG-TRIM25 was cotransfected with constructs encoding full-length NS1-B or NS1-B truncations into 293T cells for 24 h. Whole-cell lysates were subjected to immunoprecipitation with an anti-FLAG antibody, followed by IB with anti-FLAG and anti-Myc antibodies. (D) The influence of the constructs encoding full-length NS1-B or NS1-B truncations on the ubiquitination of RIG-I was confirmed by immunoprecipitation and immunoblotting assays with anti-HA, anti-FLAG, and anti-Myc antibodies. (E) The relative RIG-I ubiquitination levels with overexpression of full-length NS1-B and NS1-B truncations (lanes 4 to 8 from left in panel D) were quantitated by densitometry and normalized to the corresponding full-length NS1-B or NS1-B truncations. The error bars represent the SE.