Table 1.
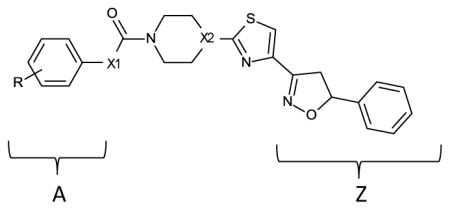
| |||||
|---|---|---|---|---|---|
| Compound | X1 | X2 | R | Apparent IC50 (nM) | Ki (pM) estimate |
| 1a | O | CH | H | 0.07 | 6.3 |
| 1b | O | CH | 4-CN | 0.14 | 12.8 |
| 1c | O | CH | 3-CN | 0.03 | 2.5 |
| 1d | O | CH | 4-Cl | 0.08 | 7.2 |
| 1e | O | CH | 4-CF3 | 0.36 | 33.6 |
| 1f | O | CH | 2-CL | 0.06 | 5.3 |
| 1g | O | CH | 3,5-CH3 | 42 | 3962 |
| 1h | O | CH | 2-CH3 | 0.04 | 3.4 |
| 1i | O | CH | 2,5-CH3 | 35 | 3302 |
| 1j | O | CH | 3-CL | 0.41 | 38.3 |
| 1k | O | CH | 2,6-CH3 | 2000 | 188,680 |
| 1l | O | CH | 3-CH3 | 0.6 | 56.3 |
| 1m | O | CH | 4-CH3 | 0.2 | 18.5 |
| 1n (notea) | O | CH | H | 29 | 2735 |
| 1o (noteb) | O | CH | H | 0.02 | INDh |
| 1p | O | CH | 4-OCH3 | 0.01 | INDh |
| 2a | NH | CH | 2-CH3 | 600 | 56,603 |
| 2b (notec) | NH | CH | H | 222 | 20,943 |
| 2c | NH | CH | H | 190 | 17,924 |
| 2d (noted) | NH | CH | NAg | 382 | 36,037 |
| 2e (notee) | NH | CH | H | 9.8 | 924 |
| 2f (notef) | NH | CH | H | 25 | 2358 |
| 3a | O | N | H | 1.56 | 147 |
| 3b | O | N | 4-CN | 0.09 | 8.1 |
| 3c | O | N | 3-CN | 0.2 | 18.5 |
| 3d | O | N | 4-CL | 0.01 | INDh |
| 3e | O | N | 4-CF3 | 0.16 | 14.7 |
| 3f | O | N | 4-NO2 | 0.2 | 18.5 |
fragment Z =
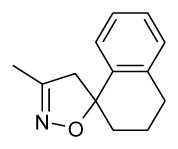
fragment A =
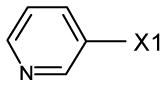
fragment A =
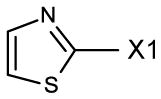
fragment A =
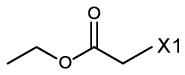
fragment A =
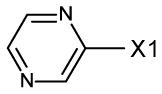
fragment A =
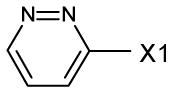
NA(not applicable)
IND(indeterminable)
IC50 values were determined as described in Experimental Procedures. Reactions were measured over 40 min, but because inhibition was time dependent, only initial linear rates were used (0 to about 25min).
