Abstract:
The dysfunction of endothelial progenitor cells (EPCs) was found to be associated with vascular complications in diabetes mellitus (DM) patients. Previous studies found that regular exercise could improve the function of EPCs in DM patients, but the underling mechanism was unclear. Irisin, a newly identified myokine, was induced by exercise and has been demonstrated to mediate some of the positive effects of exercise. In this study, we hypothesize that irisin may have direct effects on EPC function in DM mice. These data showed for the first time that irisin increased the number of EPCs in peripheral blood of DM mice and improved the function of EPCs derived from DM mice bone marrow. The mechanism for the effect of irisin is related to the PI3K/Akt/eNOS pathway. Furthermore, irisin was demonstrated to improve endothelial repair in DM mice that received EPC transplants after carotid artery injury. The results of this study indicate a novel effect of irisin in regulating the number and function of EPCs via the PI3K/Akt/eNOS pathway, suggesting a potential for the administration of exogenous irisin as a succedaneum to improve EPC function in diabetic patients who fail to achieve such improvements through regular exercise.
Key Words: endothelial progenitor cells, irisin, diabetes mellitus, proliferation, migration, endothelial repairing
INTRODUCTION
Endothelial progenitor cells (EPCs) are a pool of circulating bone marrow–derived progenitor cells1 that display the potential for endothelial repair2,3 and neovascularization.1 Unfortunately, reduced number and function of EPCs are prevalent in patients with diabetes mellitus (DM).4,5 As an association between endothelial dysfunction and vascular complications in DM is well established, several studies have tried to restore the dysfunction of EPCs mediated by DM. For example, in patients with type 1 DM, the restoration of normoglycemia by islet transplantation improved EPC number and function effectively.6 Insulin increased EPC colony forming units and tube formation in vitro7 and increased the number of EPCs in type 2 DM patients.8 Metformin,9 sulfonylureas,10 and thiazolidinediones11 also increased circulating EPCs in patients with type 2 DM.
Although previous data from experimental and clinical studies suggested that therapies designed to control hyperglycemia play a role in improving EPC function, the efficiency of these therapies was not satisfactory. More importantly, whether these therapies can prevent microvascular and macrovascular complications in DM is also unknown. Thus, it is necessary to further elucidate the mechanism of EPC dysfunction in DM patients and find a more effective therapeutic and preventive strategy to prevent microvascular and macrovascular complications.
Irisin, a proteolytic hormone derivative of the fibronectin type III domain containing 5 (fndc5) gene, is a newly discovered hormone secreted by myocytes.12 Irisin is induced by exercise and has been demonstrated to stimulate the browning of white adipocytes and thermogenesis.13 Thus, irisin has been proposed to be a bridge between exercise and metabolic homeostasis. In a previous study, it was reported that overexpression of irisin increased glucose tolerance and alleviated insulin resistance in high fat-fed mice.12 In type 2 DM patients, serum irisin was found to be negatively associated with hyperglycemia,14 and the level of serum irisin in type 2 DM patients was lower than in the nondiabetic population.15 More interestingly, in addition to the regulatory effects on fat and glucose metabolism, irisin demonstrated the potential to promote mouse H19-7 HN cell16 and human endothelial cell17 proliferation. These data suggest that irisin can exert a pro-proliferation effect in addition to its role in regulating metabolic homeostasis. However, the role of irisin in regulating EPCs in DM is unclear.
In this study, we treated DM mice with human recombinant irisin to observe its effects on EPC number. Furthermore, we treated EPCs isolated from DM mice with irisin to observe its effects on EPC function, and the possible signaling mechanisms were characterized. EPCs were transplanted into a mouse carotid artery injury model to determine whether irisin might improve the endothelial repair function of EPCs.
MATERIALS AND METHODS
Animal Care and Treatment
A total of 60 male C57/BL6 mice (4 weeks old; 13–15 g; purchased from Daping Hospital, Third Military Medical University) were selected at random. The mice were raised with free access to food and water and exposed to a 12-hour day/night cycle. Mice were randomly divided into 4 equal groups (n = 15 per group): the normal control group (NC), the normal control + irisin group (NC-irisin), the diabetic mellitus group (DM), and the diabetic mellitus + irisin group (DM-irisin). The NC group mice received a normal diet. The DM model mice were induced by intraperitoneal injection of streptozotocin (35 mg/kg body weight; Sigma) followed by feeding with a high-fat diet (HFD) for 8 weeks. The HFD consisted of 20% protein, 45% fat, and 35% carbohydrates, as a percentage of total calories (Medicience, Yangzhou, China). The DM model was confirmed by serum glucose and triglyceride (TG) levels. The NC-irisin and DM-irisin groups were then intraperitoneally injected with irisin (0.5 mg/kg body weight; Phoenix Pharmaceuticals) once a day for 8 weeks. The NC and DM groups were intraperitoneally injected with an equivalent volume of sterile phosphate-buffered saline. At the end of the treatment, the mice were killed after anesthesia, and blood samples were collected for analysis of the EPCs and serum parameters. All animal experiments were approved by the Experimental Animal Ethics Committees of the PLA Kunming General Hospital, and the investigation conforms to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication 85-23, revised 1996).
Analysis of Blood Parameters
After 8 weeks, the mice were euthanized with pentobarbital. Blood samples were collected. Plasma glucose levels were measured using the glucose oxidase method, and serum lipid profiles, including total cholesterol, TG, and low-density lipoprotein, were measured using an enzymatic assay (Roche, Darmstadt, Germany). Serum levels of irisin were measured using an ELISA kit (Phoenix Pharmaceuticals).
Quantification of Circulating EPCs by Flow Cytometry
The frequencies of EPCs were analyzed by fluorescence activated cell sorting in freshly collected whole blood samples. The total white blood cell number was determined using a hematology analyzer (Mindray BC-5000) according to the manufacturer's instructions. EPCs were detected using antibodies directed against Sca-1 (BioLegend, San Diego, CA) and Flk-1 (BioLegend). EPCs were double positive for Sca-1 and Flk-1.18
Culture and Treatment of Bone Marrow–Derived EPCs
Bone marrow–derived EPCs were cultured as previously described.1 In brief, after killing the mice, bone marrow was isolated from the femurs and tibias and subjected to density gradient centrifugation to isolate mononuclear cells. The mononuclear cells were plated on fibronectin-coated flasks and plates. EGM-2 MV bullet Kit medium (Lonza, Switzerland) supplemented with 5% fetal bovine serum, recombinant human (rh) EGF, rhFGF-B, rhVEGF, rhIGF-1, ascorbic acid, and heparin was used to culture the EPCs. The cells that were double positive for DiI-labeled acetylated low-density lipoprotein (Molecule Probe) and Ulex europaeus agglutinin-1 (UEA-1) (Sigma) by direct fluorescent staining were identified as EPCs. The cultured cells isolated from DM mice were incubated with human recombinant irisin (100 ng/mL; Phoenix Pharmaceuticals). In another set of experiments, EPCs were incubated with irisin and NG-nitro-l-arginine methyl ester (l-NAME, 0.1 mM; Sigma).
EPC Proliferation and Migration Assay
5-Bromo-2-deoxyUridine (BrdU) incorporation was used to assess EPC proliferative activity according to the manufacturer's instructions. In brief, cells were incubated with fluorescein isothiocyanate-labeled BrdU (10 mM; Sigma) for 24 hours. Then, the cells were fixed with 4% paraformaldehyde followed by 4′, 6-diamidino-2-phenylindole (DAPI) staining. The cells positive for fluorescein isothiocyanate and DAPI were photographed by fluorescence microscopy. Pictures were analyzed with Image J software, and the proliferative ratio = BrdU-positive cells/DAPI-positive cells × 100%.
The migration of EPCs was assessed using a modified Boyden chamber assay. In brief, EPCs were resuspended in serum-free medium, and the cells (5 × 104) were placed in the upper chamber; the lower chamber contained medium with 5% fetal bovine serum and vascular endothelial growth factor (20 ng/mL). After incubation for 12 hours, the upper side of the membrane was collected, wiped with a cotton swab, and fixed with 4% paraformaldehyde, and crystal violet was used to stain the cells. The pigmenting cells, which represented the migrating cells, were counted in 6 random fields (×400) under a microscope.
Western Blot Analysis
To analyze the phospho-eNOS and phospho-Akt levels, EPCs were incubated with irisin (100 ng/mL) for different periods (0, 5, 10, 15, 30 minutes) with or without PI 3-kinase (PI3K) inhibitor (LY-294002, 1 μM; Sigma). Total protein was extracted from cultured EPCs after treatment as described above by cell lysis buffer (Beyotime, Shanghai, China) supplemented with 0.5 mM Phenylmethanesulfonyl fluoride and 2 mM sodium orthovanadate. Protein concentration was assayed using the BCA analysis kit (Beyotime). Equal amounts of proteins (30–40 μg) were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis and then transferred onto polyvinylidene fluoride membranes. After blocking with 5% BSA, the membranes were probed with specific antibodies against eNOS, p-eNOS (Ser1177), Akt, p-Akt (Ser473) (Cell Signaling), and β-actin (Beyotime). Bands were scanned and analyzed using the ImageQuant TL system.
Carotid Artery Injury and EPC Transplant Model
The carotid artery injury model was established as described previously.19 In brief, DM mice were anesthetized by intraperitoneal administration of 50 mg/kg of pentobarbital. A midline incision of the ventral side of the neck was made, and the left carotid artery was exposed. The proximal side of the common carotid artery and distal side of the internal and external carotid artery were temporarily ligatured. A flexible 0.014″ PTCA guide wire (Abbott Vascular) was curved, introduced to the proximal side of the right site of the external carotid artery, and passed 3 times along the common carotid artery in a rotating manner. After removal of the wire, the proximal ligature of the external carotid artery was tied off, and the skin was closed. The mice were then divided to 3 groups: DM mice transplanted with EPCs (5 × 103/mL, 1 mL) isolated from NC mice (NC-EPCs), DM mice transplanted with EPCs (5 × 103/mL, 1 mL) isolated from DM mice (DM-EPCs), and DM mice transplanted with EPCs (5 × 103/mL, 1 mL) isolated from DM mice and injection with irisin (0.5 mg/kg body weight) (DM-EPCs + irisin). Animals were euthanized 2 weeks after the induction of carotid artery injury.
Assessment of Endothelial Repair
The whole-mount carotid arteries of mice were collected 2 weeks after carotid artery injury. Re-endothelialization was assessed by staining the artery with 0.5% Evans blue dye (Sigma) as described previously.20 The areas of Evans blue staining represented the nonendothelialized artery. Pictures were photographed using a digital camera and analyzed using the ImageJ software. For the neointimal thickening assessment, cross sections of artery underwent hematoxylin and eosin staining, and the sections were photographed under a Nikon E600 microscope. The medial area, neointimal area, and neointima/media ratio of 6 sections per mouse were analyzed using the ImagePro-Plus software.
Statistical Analysis
All data were presented as the means ± SE. SPSS 16.0 was used for statistical analysis. Intergroup comparisons were performed by 1-way analysis of variance accompanied by the post hoc Tukey's test. Comparisons between 2 treatment groups were analyzed by Student's t test. Probability values of P < 0.05 were considered statistically significant.
RESULTS
Irisin Increased the Number of Circulating EPCs in DM Mice
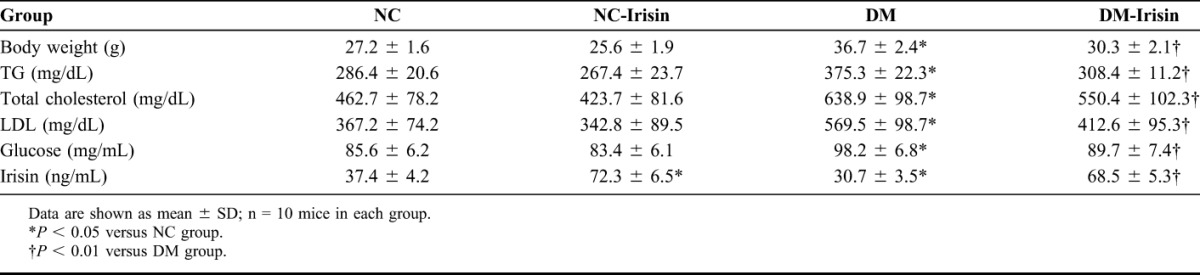
After feeding with HFD for 8 weeks, mice in the DM group exhibited decreased serum irisin levels and increased body weight, serum TG levels, and plasma glucose levels compared with the NC group (P < 0.05). Treatment with recombinant irisin increased the serum irisin levels in both NC-irisin and DM-irisin mice (P < 0.01). In DM-irisin mice, the body weight, serum TG level, and plasma glucose level were significantly lower than those in DM mice (P < 0.05). However, there were no significant differences in these parameters between the NC and NC-irisin groups (Table 1).
TABLE 1.
Body Weight and Blood Parameters of Mice in Different Groups
Based on a previous study, the cells double positive for Sca-1 and Flk-1 were recognized as EPCs (Sca-1+Flk-1+EPCs) in peripheral blood in this study. As shown in Figure 1, the number of Sca-1+Flk-1+ EPCs was significantly lower in DM mice than in NC mice (P < 0.05). The level of Sca-1+Flk-1+ EPCs was increased slightly in the peripheral blood of mice in the NC-irisin group but increased robustly in the DM-irisin group (P < 0.05).
FIGURE 1.
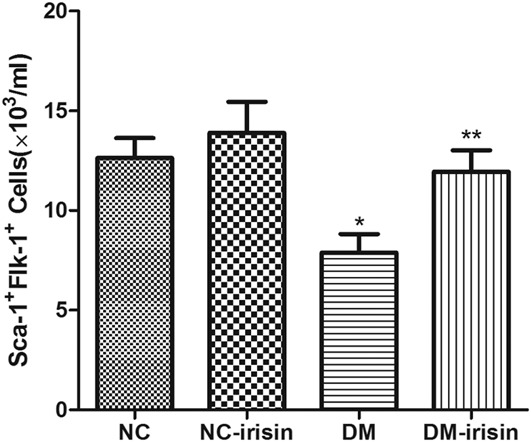
Irisin increased the number of Sca-1+Flk-1+ EPCs in DM mice. In DM mice, the number of Sca-1+Flk-1+ EPCs in peripheral blood was reduced significantly compared with NC mice. Treatment with irisin increased the number of Sca-1+Flk-1+ EPCs significantly in DM mice. *P < 0.05 versus NC group, **P < 0.05 versus DM group (n = 6 in each group).
Irisin Improves Proliferation and Migration of Bone Marrow–derived EPCs in DM Mice
The change of Sca-1+Flk-1+ EPC level in the peripheral blood suggested that irisin might improve EPC mobilization or proliferation in DM mice. Thus, in this part, the effects of irisin on the proliferation and migration of bone marrow–derived EPCs were examined. As shown in Figure 2, the proliferation of EPCs isolated from DM mice decreased by 26% (P < 0.05), and the migration of EPCs decreased by 32% (P < 0.05) compared with NC mice. In cells isolated from control mice, after incubation with irisin, the proliferation and migration only increased slightly. However, in cells isolated from DM mice, the proliferation and migration increased robustly (proliferation increased by 50.3% and migration by 60.8%, P < 0.05). The effect of irisin on proliferation and migration can be diminished by the eNOS inhibitor l-NAME (Fig. 2). These results suggested that the effect of irisin in promoting the proliferation and migration of EPCs in DM mice might operate through the regulation of eNOS.
FIGURE 2.
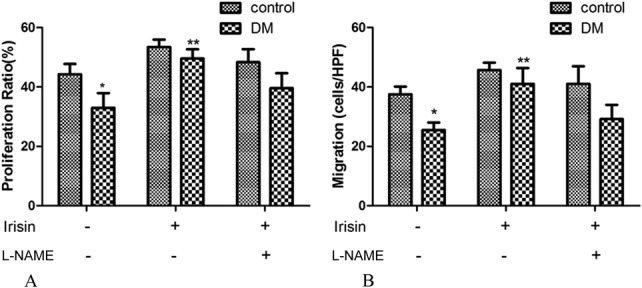
Irisin improved the proliferative and migratory capacities of bone marrow–derived EPCs. Both proliferation (A) and migration (B) were decreased in EPCs derived from DM mice compared with NC mice. Irisin slightly increased the proliferation and migration of EPCs derived from NC mice but significantly increased the values for EPCs derived from DM mice. The effect of irisin on proliferation and migration can be inhibited by L-NAME. *P < 0.05 versus NC group, **P < 0.05 versus DM group. Cells were harvested from 3 mice in each group.
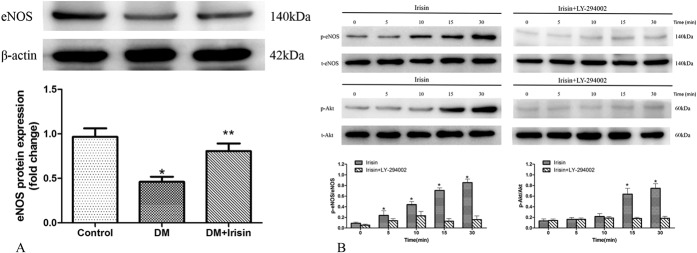
Irisin Increased eNOS Expression and Phosphorylation of EPCs in Part by PI3K/Akt Pathway
Previous studies revealed that eNOS played a critical role in regulating EPC function, and our results above suggested that eNOS might be the critical medium between irisin and EPC function. Thus, in this part, the effect of irisin on eNOS was observed. After incubation with irisin, the expression of eNOS protein in EPCs isolated from DM mice was significantly increased (Fig. 3A). The phosphorylation of eNOS at Ser1177 also increased after incubation with irisin (Fig. 3B), as did the phosphorylation at Ser473 of Akt, the upstream enzyme of eNOS. Given that Akt is activated by PI3K, the PI3K inhibitor (LY-294002) was used and was shown to inhibit irisin-induced eNOS and Akt phosphorylation (Fig. 3B). These results suggested that irisin could regulate EPC function through the PI3K/Akt/eNOS pathway.
FIGURE 3.
Irisin increased the expression of EPCs and induced the phosphorylation of eNOS through the PI3K/Akt pathway. A, Expression of eNOS protein was decreased in EPCs derived from DM compared with NC mice, and treatment with irisin increased eNOS expression in DM mice. *P < 0.05 versus NC group, **P < 0.05 versus DM group (n = 3). B, Representative Western blots for p-eNOS and p-Akt from EPCs stimulated with irisin alone and with irisin following incubation with the PI3K inhibitor LY-294002 for 30 minutes. Irisin induced the phosphorylation of eNOS and Akt, and these effects can be inhibited by LY-294002. *P < 0.05 versus control group (n = 3).
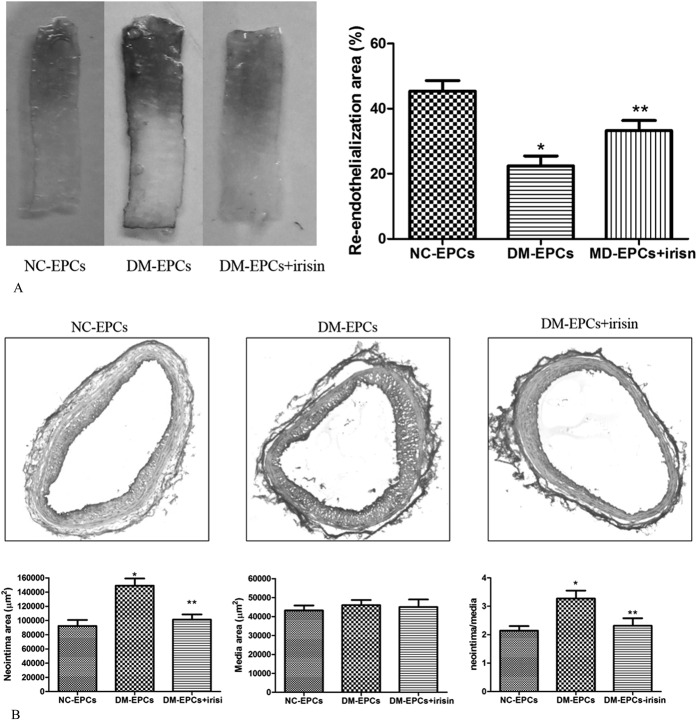
Irisin Promoted the Endothelial Repair of EPCs in DM Mice
In whole-mount carotid arteries, Evans blue staining indicated that the re-endothelialization was decreased in mice transplanted with EPCs isolated from DM mice compared with mice transplanted with EPCs isolated from NC mice (P < 0.05). However, treatment with irisin improved re-endothelialization in mice transplanted with EPCs isolated from DM mice (P < 0.05) (Fig. 4A). Accordingly, the formation of neointima was increased in mice transplanted with EPCs isolated from DM mice compared with mice transplanted with EPCs isolated from NC mice (P < 0.05), and irisin significantly decreased the formation of neointima in mice transplanted with EPCs isolated from DM mice (P < 0.05) (Fig. 4B). These results suggested that irisin improved the endothelial repair function of EPCs in DM mice.
FIGURE 4.
Irisin improved endothelial repair in DM mice. A, Evans blue staining represented re-endothelialization in DM mice transplanted with EPCs after carotid artery injury. Quantification of re-endothelialization area showed that re-endothelialization was decreased in mice transplanted with DM-EPCs compared with NC-EPCs, and irisin improved re-endothelialization in mice transplanted with DM-EPCs. *P < 0.05 versus NC-EPCs, **P < 0.05 versus DM-EPCs (n = 3). B, Irisin prevents neointima formation. Representative hematoxylin and eosin staining of the carotid artery of mice transplanted with EPCs after carotid artery injury. Quantitative morphometry showed that the neointima area and the neointima/media ratio were higher in mice transplanted with DM-EPCs compared with NC-EPCs, and treatment with irisin inhibited the formation of neointima in mice transplanted with DM-EPCs. *P < 0.05 versus NC-EPCs, **P < 0.05 versus DM-EPCs; 6 sections per mouse, 3 arteries harvested from 3 mice in each group.
DISCUSSION
Endothelial dysfunction is the initial factor of vascular disease; to improve the function of endothelial lining is critical for slowing or reversing the progression of vascular disease. Previous studies suggest that EPCs can restore the damaged endothelial lining.2,3,21,22 Reduced number and availability of EPCs are responsible for the occurrence of endothelial dysfunction and vascular complications in some chronic diseases, such as diabetes. The present study in DM mice also showed the reduced number and deficient function of EPCs, which is consistent with the previous studies.4,5,18 Although the dysfunction of EPCs was found to be associated with vascular complications in diabetic patients,5 there remained few efficient treatment strategies for this problem. Previous studies found that regular exercise could improve the function of EPCs.23,24 However, no data showed the direct link between exercise and EPC function. Irisin, a newly identified hormone secreted by myocytes, has been demonstrated to mediate some of the positive effects of exercise. In this study, we hypothesize that irisin might have direct effects on EPC function in DM mice. These data showed the first evidence that irisin increased the number of EPCs in the peripheral blood of DM mice. Irisin also increased the number of EPCs in normal mice, although with lower efficiency. This difference may contribute to the complications of the effect of irisin. We speculated in vivo that, irisin not only regulates the number of EPCs directly but may also act indirectly through regulating the glucose and TG metabolism.
In DM patients and animals, not only the number of EPCs was decreased but the function of EPCs was also found to be deficient.18,25 Thus, we observed the effect of irisin on the function of EPCs. The presented data showed that irisin improved the proliferative and migratory capacities of EPCs derived from DM mouse bone marrow in vitro. In vivo, irisin improved endothelial repairing in DM mice, which were transplanted with EPCs. These results suggested that irisin has the direct effect on EPCs' proliferation and migration. Most important is that irisin may have the effect on endothelial repairing by improving EPCs' function or the other mechanisms.
Previous studies showed that eNOS was the key regulator involved in the proliferation and migration of EPCs26; thus, we investigated the effect of irisin on eNOS and found the effect of irisin on EPCs was eNOS dependently and irisin regulated the expression of eNOS. Given that phosphorylation of Ser1177 by Akt is critical for activation of eNOS, we investigated the effects of irisin in this regard. The data show that irisin can increase the phosphorylation of Ser1177 and Akt of EPCs. Furthermore, inhibition of PI3K by LY-294002 can inhibit irisin-induced phosphorylation of eNOS and Akt, indicating that irisin participate in the activation of the PI3K/Akt pathway. Interestingly, recent study by Han et al27 found that irisin can induce eNOS phosphorylation via AMPK/Akt pathway in HUVECs, whereas Song et al17 found that irisin can effectively promote HUVECs proliferation by activating the ERK signaling pathway rather than Akt pathway. The different signaling pathway activated by irisin may attribute to the different cell types. In addition, the specific receptor of irisin and the exact mechanism of interaction between irisin and cells were unclear; thus, the signaling pathway activated by irisin maybe multicomponent.
In conclusion, this study found that irisin improved EPC number and function in DM mice. The mechanism for the effect of irisin is related to the PI3K/Akt/eNOS pathway. This result suggests a potential for the administration of exogenous irisin as a succedaneum to improve EPC function for diabetic patients who fail to improve through regular exercise.
ACKNOWLEDGMENTS
The authors thank MD Huali Kang (Cardiovascular Institute of Xinqiao Hospital, Third Military Medical University, Chongqing, People's Republic of China), Qing Zhou (Department of Protective Medicine, Institute of combined Injury, Third Military Medical University, Chongqing, People's Republic of China), and Xiaohong Wang (Institute of Traffic Medicine, Third Military Medical University, Chongqing, People's Republic of China) for their excellent technical assistance.
Footnotes
Supported by Grants from the National Natural Science Foundation of China (81170316).
The authors report no conflicts of interest.
G. Zhu and M. Song contributed equally to this work.
REFERENCES
- 1.Asahara T, Murohara T, Sullivan A, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. [DOI] [PubMed] [Google Scholar]
- 2.Griese DP, Ehsan A, Melo LG, et al. Isolation and transplantation of autologous circulating endothelial cells into denuded vessels and prosthetic grafts: implications for cell-based vascular therapy. Circulation. 2003;108:2710–2715. [DOI] [PubMed] [Google Scholar]
- 3.Walter DH, Rittig K, Bahlmann FH, et al. Statin therapy accelerates reendothelialization: a novel effect involving mobilization and incorporation of bone marrow-derived endothelial progenitor cells. Circulation. 2002;105:3017–3024. [DOI] [PubMed] [Google Scholar]
- 4.Hill JM, Zalos G, Halcox JP, et al. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003;348:593–600. [DOI] [PubMed] [Google Scholar]
- 5.Fadini GP, Miorin M, Facco M, et al. Circulating endothelial progenitor cells are reduced in peripheral vascular complications of type 2 diabetes mellitus. J Am Coll Cardiol. 2005;45:1449–1457. [DOI] [PubMed] [Google Scholar]
- 6.Petrelli A, Maestroni A, Fadini GP, et al. Improved function of circulating angiogenic cells is evident in type 1 diabetic islet-transplanted patients. Am J Transplant. 2010;10:2690–2700. [DOI] [PubMed] [Google Scholar]
- 7.Humpert PM, Djuric Z, Zeuge U, et al. Insulin stimulates the clonogenic potential of angiogenic endothelial progenitor cells by IGF-1 receptor-dependent signaling. Mol Med. 2008;14:301–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fadini GP, de Kreutzenberg SV, Mariano V, et al. Optimized glycaemic control achieved with add-on basal insulin therapy improves indexes of endothelial damage and regeneration in type 2 diabetic patients with macroangiopathy: a randomized crossover trial comparing detemir versus glargine. Diabetes Obes Metab. 2011;13:718–725. [DOI] [PubMed] [Google Scholar]
- 9.Liao YF, Chen LL, Zeng TS, et al. Number of circulating endothelial progenitor cells as a marker of vascular endothelial function for type 2 diabetes. Vasc Med. 2010;15:279–285. [DOI] [PubMed] [Google Scholar]
- 10.Chen LL, Liao YF, Zeng TS, et al. Effects of metformin plus gliclazide compared with metformin alone on circulating endothelial progenitor cell in type 2 diabetic patients. Endocrine. 2010;38:266–275. [DOI] [PubMed] [Google Scholar]
- 11.Esposito K, Maiorino MI, Di Palo C, et al. Effects of pioglitazone versus metformin on circulating endothelial microparticles and progenitor cells in patients with newly diagnosed type 2 diabetes–a randomized controlled trial. Diabetes Obes Metab. 2011;13:439–445. [DOI] [PubMed] [Google Scholar]
- 12.Bostrom P, Wu J, Jedrychowski MP, et al. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481:463–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lecker SH, Zavin A, Cao P, et al. Expression of the irisin precursor FNDC5 in skeletal muscle correlates with aerobic exercise performance in patients with heart failure. Circ Heart Fail. 2012;5:812–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kurdiova T, Balaz M, Vician M, et al. Effects of obesity, diabetes and exercise on Fndc5 gene expression and irisin release in human skeletal muscle and adipose tissue: in vivo and in vitro studies. J Physiol. 2014;592:1091–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu JJ, Wong MD, Toy WC, et al. Lower circulating irisin is associated with type 2 diabetes mellitus. J Diabetes Complications. 2013;27:365–369. [DOI] [PubMed] [Google Scholar]
- 16.Moon HS, Dincer F, Mantzoros CS. Pharmacological concentrations of irisin increase cell proliferation without influencing markers of neurite outgrowth and synaptogenesis in mouse H19-7 hippocampal cell lines. Metabolism. 2013;62:1131–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Song H, Wu F, Zhang Y, et al. Irisin promotes human umbilical vein endothelial cell proliferation through the ERK signaling pathway and partly suppresses high glucose-induced apoptosis. PLoS One. 2014;9:e110273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Westerweel PE, Teraa M, Rafii S, et al. Impaired endothelial progenitor cell mobilization and dysfunctional bone marrow stroma in diabetes mellitus. PLoS One. 2013;8:e60357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Strehlow K, Werner N, Berweiler J, et al. Estrogen increases bone marrow-derived endothelial progenitor cell production and diminishes neointima formation. Circulation. 2003;107:3059–3065. [DOI] [PubMed] [Google Scholar]
- 20.Krasinski K, Spyridopoulos I, Asahara T, et al. Estradiol accelerates functional endothelial recovery after arterial injury. Circulation. 1997;95:1768–1772. [DOI] [PubMed] [Google Scholar]
- 21.Dimmeler S, Zeiher AM. Vascular repair by circulating endothelial progenitor cells: the missing link in atherosclerosis? J Mol Med (Berl). 2004;82:671–677. [DOI] [PubMed] [Google Scholar]
- 22.Murohara T, Ikeda H, Duan J, et al. Transplanted cord blood-derived endothelial precursor cells augment postnatal neovascularization. J Clin Invest. 2000;105:1527–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laufs U, Urhausen A, Werner N, et al. Running exercise of different duration and intensity: effect on endothelial progenitor cells in healthy subjects. Eur J Cardiovasc Prev Rehabil. 2005;12:407–414. [DOI] [PubMed] [Google Scholar]
- 24.Sandri M, Adams V, Gielen S, et al. Effects of exercise and ischemia on mobilization and functional activation of blood-derived progenitor cells in patients with ischemic syndromes: results of 3 randomized studies. Circulation. 2005;111:3391–3399. [DOI] [PubMed] [Google Scholar]
- 25.Kuliszewski MA, Ward MR, Kowalewski JW, et al. A direct comparison of endothelial progenitor cell dysfunction in rat metabolic syndrome and diabetes. Atherosclerosis. 2013;226:58–66. [DOI] [PubMed] [Google Scholar]
- 26.Everaert BR, Van Craenenbroeck EM, Hoymans VY, et al. Current perspective of pathophysiological and interventional effects on endothelial progenitor cell biology: focus on PI3K/AKT/eNOS pathway. Int J Cardiol. 2010;144:350–366. [DOI] [PubMed] [Google Scholar]
- 27.Han F, Zhang S, Hou N, et al. Irisin improves endothelial function in obese mice through the AMPK-eNOS pathway. Am J Physiol Heart Circ Physiol. 2015;309:H1501–H1508. [DOI] [PubMed] [Google Scholar]