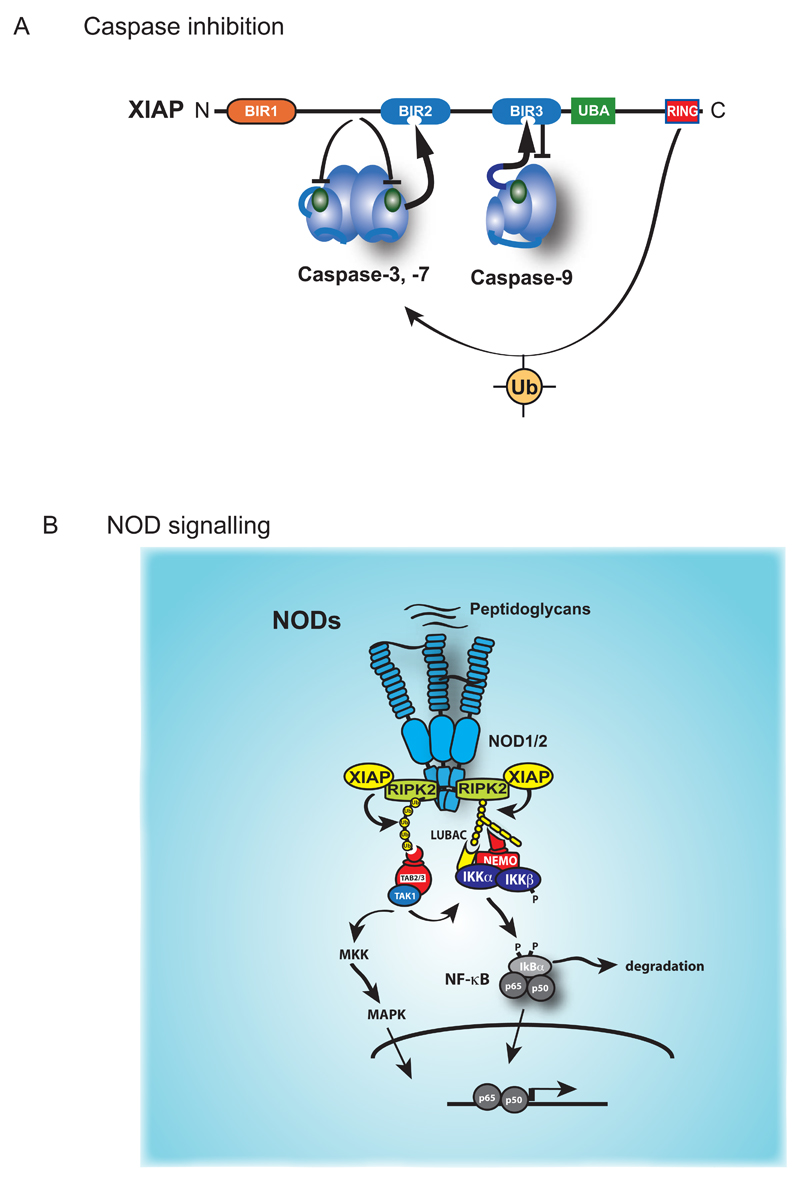
Figure 5. XIAP-mediated regulation of caspases and NF-κB.
(A) XIAP directly inhibits the effector caspase-3 and caspase-7, and the initiator caspase-9. The sequence preceding the BIR2 domain of XIAP occupies the catalytic pocket of caspase-3 or caspase-7, thereby blocking substrate entry. In addition, the BIR2 domain interacts with the IBM of caspase-3 or caspase-7 that is exposed following their proteolytic activation (shown as an arrow). XIAP-mediated inhibition of caspase-9 requires proteolytic cleavage of caspase-9, which exposes an IBM that binds to the BIR3 of XIAP. Caspase-9 activity is blocked because XIAP prevents caspase 9 dimerization, a prerequisite for initiator caspasenactivity. The RING domain of XIAP also contributes to caspase inhibition. (B) NOD-mediated activation of NF-kB and MAPK signalling. Detection of bacterial peptidoglycans by NOD1 and NOD2 results in the formation of an oligomeric signalling complex that recruits RIPK2, and XIAP. XIAP mediated ubiquitylation of RIPK2 allows the recruitment of TAB2/TAB3/TAK1 and IKKs, thereby triggering NF-kB and MAPK signalling.