Abstract
Importance
Optical coherence tomography (OCT) has transformed the clinical management of a myriad of ophthalmic conditions. Applying OCT to ophthalmic surgery may have implications for surgical decision-making and patient outcomes.
Objective
To assess the feasibility and impact on surgical-decision making of microscope-integrated intraoperative OCT (iOCT) system.
Design, Setting, and Participants
DISCOVER is an IRB-approved single-site multi-surgeon investigational device prospective consecutive case series. Participants included subjects undergoing ophthalmic surgery. Clinical characteristics were collected and iOCT imaging was obtained during surgical milestones as directed by the operating surgeon. A surgeon questionnaire was issued to each surgeon and completed after each case to evaluate the role of iOCT during surgery and its particular role in select surgical procedures. This report highlights the 1-year results (March 2014-February 2015) of the RESCAN 700 portion of the study.
Main outcomes measures
Percentage of cases with successful acquisition of iOCT imaging (i.e., feasibility). Percentage of cases that iOCT imaging altered surgical decision-making (i.e., utility)
Results
During year-1 of the DISCOVER study, 227 total eyes were enrolled (91 anterior segment cases and 136 posterior segment) to undergo imaging with the RESCAN 700 system. Successful imaging (e.g., the ability to acquire an OCT image of the tissue of interest) was obtained in 224 of 227 eyes (99%). During lamellar keratoplasty, the iOCT data provided information that altered surgeon decision-making in 38% of the cases (e.g., complete graft apposition when the surgeon believed there was interface fluid). In membrane peeling procedures, iOCT information was discordant with surgeon impression of membrane peel completeness in 19% of cases (e.g. lack of residual membrane, presence of occult membrane) impacting additional surgical maneuvers.
Conclusions and Relevance
The DISCOVER study demonstrates the feasibility of real-time iOCT with a microscope-integrated iOCT system for ophthalmic surgery. The information gained from iOCT appears to allow surgeons to assess subtle details in a unique perspective from standard en face visualization which can affect surgical decision-making some of the time, although the impact of these changes in decision-making on outcomes remains unknown. A prospective randomized masked trial is needed to confirm these results.
Introduction
Optical coherence tomography (OCT) has evolved from an experimental instrument to a critical clinical diagnostic modality and may have the potential to become a seamless surgical-guidance tool. Recent literature studies examining intraoperative OCT (iOCT) support the potential role for iOCT in ophthalmic surgery.1-10 These studies have examined the role for iOCT in multiple procedures and conditions, including epiretinal membranes, macular hole, vitreomacular traction, retinal detachment repair, lamellar keratoplasty, and cataract surgery. 1,3-6,8 The field of OCT-guided surgery remains a young and emerging field. Many early reports were retrospective with small sample sizes.3-6,8 The PIONEER study, examining the utility of a microscope attached external OCT device during ophthalmic surgery, provided the first large prospective data set to examine the feasibility and utility of iOCT.1
However, the vast majority of previous studies focused on systems that were not integrated into the OCT that did not allow for real-time feedback or heads-up surgeon interfaces. The future of iOCT will likely be founded in integrative technologies. New systems are now emerging that provide the surgeon with microscope-integrated technology and may enable rapid “real-time” feedback on the anatomic changes that occur during surgical manipulations.6,11-14 The key features of these systems in maximizing outcomes and minimizing surgical disruption remain unclear, as well as the specific procedures that would benefit from microscope-integrated iOCT.
In order to better understand the feasibility and utility of microscope integrated iOCT, the Determination of feasibility of Intraoperative Spectral domain microscope Combined/integrated OCT Visualization during En face Retinal and ophthalmic surgery (DISCOVER) study was initiated. This report provides the 1-year results for the assessment of feasibility and utility (i.e., impact on surgical decision-making) of microscope-integrated iOCT for ophthalmic surgery for one of the prototypes in the DISCOVER study, the RESCAN 700 (Carl Zeiss Meditec, Oberkochen, Germany).
Methods
DISCOVER (Determination of feasibility of Intraoperative Spectral domain microscope Combined/integrated OCT Visualization during En face Retinal and ophthalmic surgery) is a Cleveland Clinic IRB-approved prospective multi-surgeon investigational device study. The study adhered to the tenets of the Declaration of Helsinki. A written, informed consent was obtained from all patients participating in DISCOVER.
The study included an intraoperative protocol for imaging during surgical milestones and immediate surgeon feedback. Data variables collected included indication for surgery, procedure, visual acuity, ocular comorbidities, details regarding surgical maneuvers/techniques (e.g., instrument type, surgical approach), type of OCT imaging obtained during surgery, and adverse events. Although scheduled study visits were completed following the first postoperative visit, IRB-approval includes an additional 2-year period of postoperative review of clinical variables, imaging outcomes, and clinical outcomes.
The DISCOVER study includes 3 microscope-integrated OCT prototypes: the RESCAN 700, the Bioptigen integrated prototype (Bioptigen, Research Triangle Park, NC), and an internally developed Cole Eye integrated prototype (Cleveland Clinic, Cleveland, OH). This report will focus on the RESCAN 700 results during year 1 (i.e., March 2014-February 2015). The imaging protocol directed surgeons to obtain imaging during and/or after surgical milestones, as determined by the operating surgeon. The RESCAN 700 system includes a microscope-integrated OCT system, as previously described.2 Imaging data was reviewed by the surgeon intraoperatively and was reviewed independently postoperatively. Surgeons visualized the OCT data stream through the oculars utilizing the heads-up display or with an external display monitor, based on their preference. A research coordinator assisted with OCT acquisition, collecting surgeon feedback, and data collection.
Prespecified surgeon feedback questionnaires were completed for all cases focusing on several specific areas of interest related to the microscope-integrated system and surgical procedure, including the perceived value of iOCT to the procedure (e.g., impact on surgical decision-making), preferred ergonomics of the system (e.g., heads-up display, review mode), and issues related to workflow (e.g., interference with the case). Additionally, in select prespecified procedures (e.g., membrane peeling procedures, lamellar keratoplasty) an additional feedback form was completed related to the value of iOCT for that specific procedure.
Results
Clinical Demographics
At 1-year, 227 eyes were enrolled in the RESCAN 700 Arm of the DISCOVER study, Table 1. There were 121 females (53%) and 106 males (47%). The mean age of patients in the study was 62 years. (range:19-91 ). Overall, successful acquisition of iOCT images was obtained in 224 of 227 [99%; 95% Confidence Interval (95%CI): 98-100%] eyes. iOCT images were not obtained in 2 patients due to surgeon-decision to not image and 1 case was not imaged due to software malfunction.
Table 1.
Baseline Demographics and Clinical Characteristics of the DISCOVER Study
| Anterior | Posterior | ||||
|---|---|---|---|---|---|
| Enrollment | 91 | 136 | |||
| Eye | Right Left |
48 43 |
67 69 |
||
| Lens Status |
Pseudophakic Phakic Aphakic |
34 52 5 |
57 75 4 |
||
| Preoperative Diagnosis | Fuchs Dystrophy Bullous Keratopathy Keratoconus Failed PK Cataract Glaucoma Failed DSAEK Other |
34 (37%) 10 (11%) 8 (9%) 7 (8%) 7 (8%) 7 (8%) 5 (5%) 13 (14%) |
Epiretinal membrane Retinal detachment Proliferative diabetic retinopathy Vitreous hemorrhage Panuveitis Full-thickness macular hole Traction retinal detachment Other |
34 (25%) 28 (21%) 12 (9%) 12 (9%) 12 (9%) 10 (7%) 5 (4%) 21 (16%) |
|
| Procedures | DSAEK DALK CE/IOL DMEK Tube shunt PK Trabeculectomy Other |
43 (47%) 8 (9%) 7 (8%) 7 (8%) 4 (4%) 4 (4%) 2 (2%) 16 (18%) |
PPV Fluocinolone implant without PPV Scleral buckle without PPV Other |
126 (93%) 7 (5%) 1 (1%) 2 (1%) |
|
CE: cataract extraction; IOL: intraocular lens; PPV: pars plana vitrectomy; DSAEK: Descemet stripping automated endothelial keratoplasty; PK: penetrating keratoplasty; DALK: deep anterior lamellar keratoplasty; DMEK: Descemet membrane endothelial keratoplasty; SB: scleral buckle
Anterior Segment iOCT Summary
In the anterior segment arm, 91 eyes were enrolled. The most common procedures included were Descemet Stripping Automated Endothelial Keratoplasty (DSAEK, 43%), and Deep Anterior Lamellar Keratoplasty (DALK, 9%), Table 1. During DSAEK (Figure 1, Supplemental Video 1) and Descemet Membrane Endothelial Keratoplasty (DMEK, Figure 2), iOCT provided information related to graft position/orientation. In addition, iOCT provided visualization of interface fluid and graft/host apposition. Surgical manipulations (e.g., manual sweeping, increased air infusion pressure) were performed to minimize interface fluid (Figure 1). In DALK, iOCT provided real-time feedback of trephination depth, allowing for visualization of instrument-tissue interaction and providing immediate information related to the residual stromal bed (Figure 2). Imaging with iOCT confirmed location of intraocular implants, including glaucoma procedures (e.g., relative tube-endothelial location) and corneal inlay procedures (eFigure 1). During phacoemulsification, multiple steps of the procedure were visualized with iOCT including capsulorrhexis, hydrodissection, groove depth, and intraocular lens placement (eFigure 2).
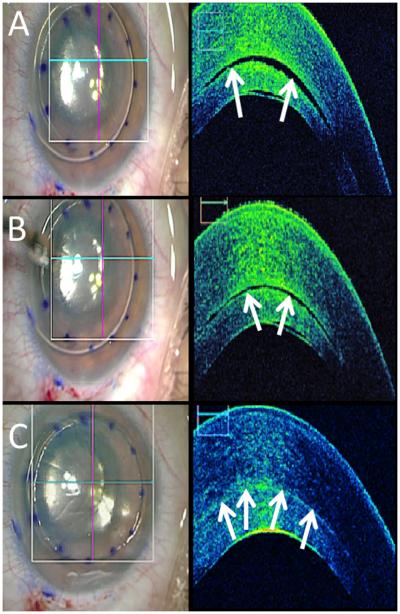
Figure 1. Time-lapse iOCT of DSAEK.
(A) En face view following air-bubble infusion (left). B-scan (right) with interface fluid (arrows). (B, C) Following corneal massage, en face view unchanged (left), but B-scans (right) reveals resolving interface fluid (arrows).
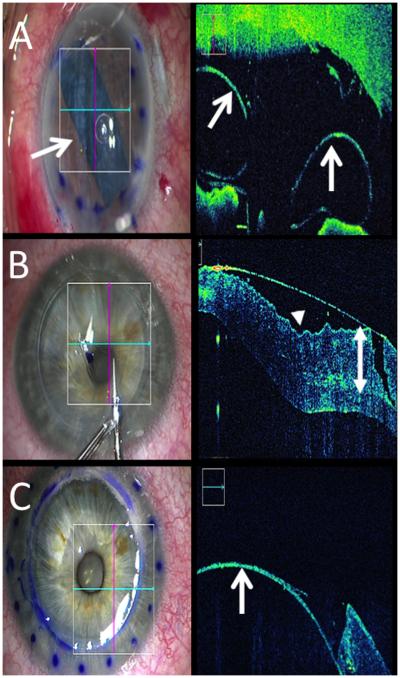
Figure 2. Lamellar keratoplasty and iOCT.
(A) iOCT confirms optimal graft orientation (arrows) follow DMEK graft placement (arrow, left). (B) iOCT reveals striae (arrowhead) and trephination depth (double arrow) during DALK. (C) Subsequent iOCT during DALK confirms dissection to Descemet membrane (arrow).
Ergonomics and Value of Microscope integrated iOCT in Anterior Segment Surgery
Surgeons reported that microscope-integrated iOCT provided valuable feedback in 82 of 91 (90%; 95%CI: 84-96%) cases. According to surgeons in the study, 40 of 91 (44%; 95%CI: 34-54%) total anterior segment cases were changed or modified due to the iOCT findings. For example, during a DSAEK procedure, iOCT revealed subclinical graft detachment in the operating room that allowed the surgeon to intervene with rebubbling prior to stopping the case. During a corneal inlay procedure, wound depth was increased to optimize implant placement. During glaucoma surgical interventions, iOCT provided valuable data in select cases on optimal tube placement (e.g., verifying sulcus placement, relative tube-cornea location, eFigure 1).
Surgeons preferred real-time iOCT feedback in 63 of 91 cases (69%; 95%CI: 60-79%), compared to 21 of 91 (23%; 95%CI: 14-32%) cases where static feedback was more optimal. The heads-up display system was preferred to the screen in 84 of 91 (92%; 95%CI: 86-97%) cases, compared to viewing the OCT information on the video display. There were no reports of the iOCT system interfering with surgery. Contamination (e.g. contaminated glove, surgical instrument) occurred in 12 of 91 (13%; 95%CI: 6-20%) anterior segment cases. None of these contaminations resulted in surgical field contamination.
Intraoperative OCT Guidance of Surgical Decision-Making: Lamellar Keratoplasty
For DSAEK cases, iOCT was noted by surgeons to provide valuable feedback in 41of 41 (100%) cases. In 17 of 41 (41%; 95%CI: 26-56%) DSAEK cases, additional maneuvers were performed based on iOCT. In DSAEK procedures, following tissue placement and prior to iOCT imaging, surgeons believed the tissue was completely apposed in 26 of 41 (63%; 95%CI: 48-78%) cases and the remaining 15 of 41 (37%; 95%CI: 22-52%) cases did not believe the tissue was completely apposed. In 5 of 26 (19%; 95%CI: 4-34%) cases that the surgeon believed the graft was fully apposed, iOCT identified persistent interface fluid, facilitating additional maneuvers during the procedure. In 7/15 (47%; 95%CI: 22-72%) cases where the surgeon did not believe the tissue to be entirely apposed, iOCT revealed complete adherence of the graft confirming apposition, minimizing surgical time and unnecessary manipulations. In 11/41 (27%; 95%CI: 13-41%) DSAEK cases, iOCT identified the absence of interface fluid, contrary to the impression of the surgeon, eliminating the need for serial sweeps during surgery and reducing operative time.
For DALK surgery, surgeons indicated the achievement of big-bubble was noted clinically and confirmed on iOCT in 3 of 8 cases (38%; 95%CI: 4-72%). iOCT identified subclinical big-bubbles in 2 of the 5 (40%; 95%CI: 0-83%) remaining cases, which guided additional maneuvers for dissection. In 3 of 8 (38%; 95%CI: 4-72%) cases, iOCT impacted both stromal resection and helped facilitate identification of dissection depth.
Posterior Segment iOCT Summary
In the posterior segment arm, 136 eyes were enrolled. The most frequent indications for surgeries were epiretinal membrane (ERM, 25%), retinal detachment (RD, 20%), proliferative diabetic retinopathy (PDR, 9%), vitreous hemorrhage (VH, 9%), and macular hole (MH, 7%), Table 1. In ERM and MH cases, iOCT identified residual membranes, allowed for visualization of tissue-instrument interactions, and confirmed completion of surgical objectives (Figure 3). Absolute shadowing was noted with real-time membrane peeling with metallic instruments (Figure 3). In select cases, true OCT-guided membrane peeling was performed when the standard view was poor due to corneal edema both with real-time visualization of tissue-instrument interactions and with identification of residual membranes that were not otherwise visible. iOCT was particularly valuable incomplex cases of membrane peeling, such as myopic foveal schisis or vitreoschisis with multi-laminar membranes (eFigure 3, Supplemental Video 2).
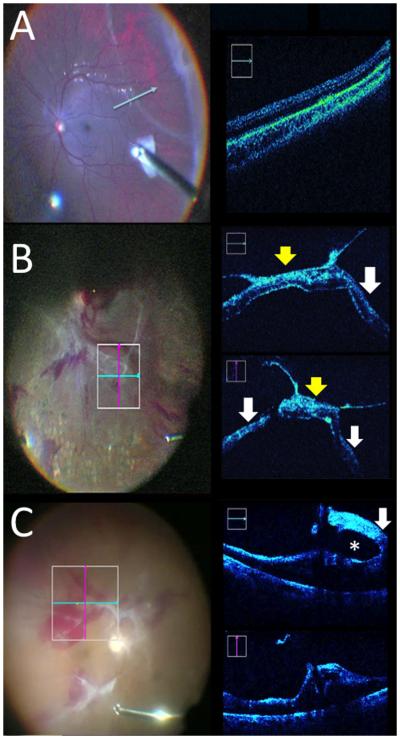
Figure 3. Real-time iOCT of membrane peeling.
(A) iOCT during peeling identifies membranes (right top and right bottom, white arrows) with shadowing from metal (yellow arrows). (B) iOCT of membrane scraper with shadowing from diamonds (yellow arrow) and membrane edge (white arrow).
During RD repair cases, iOCT provided visualization of completeness of retina/RPE apposition following perfluorocarbon liquid tamponade, as well as recurrence of subfoveal fluid after air-fluid exchange. Peripheral assessment with iOCT of retinal abnormalities facilitated discrimination between areas of subretinal fluid and white without pressure (Figure 4). In PDR cases, iOCT provided visualization of surgical planes and helped to discriminate between retinal tissue and fibrovascular scars, as well as, discriminating between traction retinal detachment and focal retinal traction (Figure 4, Supplemental Video 3). Optimal depth visualization with iOCT during choroidal biopsy was also possible (eFigure 4, Supplemental Video 4).
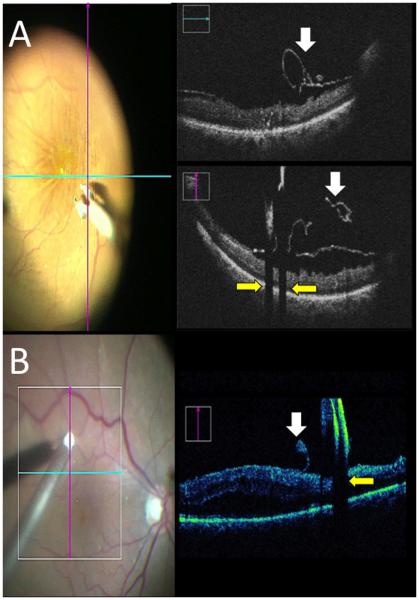
Figure 4. iOCT for RD and PDR.
(A) Possible RD (arrowheads) determined to be flat by iOCT (right). (B) PDR with iOCT-confirmed TRD (white arrows) with dense membranes (yellow arrows). (C) PDR with focal traction (white arrow) and visible surgical plane (asterisk).
Ergonomics and Value of Microscope-integrated iOCT in Posterior Segment Surgery
Overall, surgeons indicated that microscope-integrated iOCT provided valuable feedback in 97 of 136 (71%; 95%CI: 63-79%) cases. Surgeons reported that in 49 of 136 (36%; 95%CI: 28-44%) cases, information provided through iOCT resulted in changes to the surgical approach. One example during retinal detachment surgery, iOCT identified that a subretinal band in a PVR case was entirely flat under PFO when en face visualization gave the impression of elevation. Given the iOCT findings, additional membrane peeling was abandoned, minimizing surgical manipulation. A second example during another retinal detachment case, the surgeon believed there was a focal area of detachment that was confirmed by iOCT to be entirely flat in the retinal periphery (Figure 3). In a myopic schisis case, a prominent membrane was peeled from the retinal surface, but iOCT revealed a prominent persistent underlying membrane that was not perceptible to the surgeon (eFigure 3, Supplemental Video 2).
Surgeons preferred real-time iOCT feedback in 93 of 136 cases (68%; 95%CI: 60-76%), compared to 39 of 136 (29%; 95%CI: 21-37%) cases where static feedback was more optimal. The heads-up display system was preferred to the external screen in 95 of 136 (70%; 95%CI: 62-78%) cases, compared to viewing the OCT information on the video display. In 7 of 136 (5%; 95%CI: 1-9%) of cases, surgeons reported that the iOCT system interfered with surgery, including software malfunctions, microscope failure, and unresponsive foot-pedal. No adverse events occurred secondary to these issues. In 22 of 136 (16%; 95%CI: 10-22%) cases contamination during surgery (e.g. contaminated glove, surgical instrument) occurred. None of these contaminations included the surgical field.
Intraoperative OCT Guidance of Surgical Decision-Making: Membrane Peeling
In all cases of membrane peeling, indocyanine green was utilized prior to initial peeling of preretinal membranes and the internal limiting membrane. Prior to iOCT imaging, 41 of 67 (61%; 95%CI: 49-73%) cases surgeons believed membrane peeling was complete. In 9 of those 41 cases (22%; 95%CI: 9-35%), iOCT identified residual occult membranes that the surgeon determined needed additional peeling. In 26 of 67 cases (39%; 95%CI: 27-51%), the surgeons believed membrane peeling was incomplete prior to iOCT scanning. In 4 of 26 (15%; 95%CI: 1-29%) cases where the surgeon believed there was additional membrane peeling required, the iOCT informed the surgeon that membrane peeling was entirely complete and no further peeling was required. Ultimately, in 13 of 67 (19%; 95%CI: 10-28%) membrane peeling procedures, iOCT findings were discordant with surgeon impression and resulted in direct alterations to the surgical procedure.
Intraoperative OCT Guidance of Surgical Decision-Making: Retinal Detachment
Surgeons utilized iOCT in 24 cases of retinal detachment repair. Persistent subretinal fluid under perfluorocarbon liquid was identified in 17/24 (53-89%) cases. iOCT feedback that impacted surgical decision-making included identification of a MH under perfluorcarbon liquid (n=1), residual membranes requiring peeling (n=2), identification of optimal placement for drainage based on subretinal fluid (n=1), and differentiation between choroidal hemorrhage and subretinal fluid (n=1). Overall, surgeons indicated that iOCT provided feedback that altered surgeon decision-making in 5/24 (21%; 95%CI: 5-37%) cases.
Adverse Events
One serious adverse event (i.e., myocardial infarction) occurred postoperatively during the course of the study following vitrectomy surgery. The most common postoperative day 1 adverse events in both groups included corneal epithelial defects [31 of 227 (13%)] and abnormal intraocular pressure values [29 of 227 (13%)]. Seven of seven epithelial defects in the anterior segment arm occurred in eyes undergoing procedures where epithelial defects would be expected (e.g., corneal transplant, dermoid removal). In posterior segment cases, the majority of epithelial defects were in cases with more complex preoperative diagnoses (15 of 24), including proliferative diabetic retinopathy and retinal detachment. Less frequent adverse events included vitreous hemorrhage (4 of 227, 2%), fibrin (3 of 227, 1%), and hyphema (2 of 227, 1%). One partial graft dislocation occurred in a subject who underwent DSAEK surgery during the study.
Discussion
Gateway studies in real-time OCT technology are enabling transformation in ophthalmic surgical care that could facilitate image-guided surgery in ways not previously feasible. Previous studies have found compelling results for the role for iOCT in many ophthalmic surgical conditions, including both anterior and posterior procedures.1,4-6,13,15,16 This report from the DISCOVER study provides a large prospective examination of the feasibility and utility of the microscope-integrated iOCT. The rapid advancements transpiring in the iOCT field are pushing the limits to new levels of what is achievable in image-guided surgery and real-time surgeon feedback.2,6,11,14,17 Microscope-integrated iOCToffers immediate image-guidance during surgery allowing the surgeon to gauge procedural progression and completion. This live feedback may facilitate improvements in surgeon judgment, action and knowledge during procedural processes.
Although this study represents the largest prospective clinical study to date on microscope-integrated iOCT, there are important limitations that must be acknowledged. This study was non-comparative, non-randomized and was not masked. All surgeons knew that they would be using the iOCT system and this may have impacted their level of aggressiveness during the surgical procedure. Additionally, this report has focused on the intraoperative and early postoperative implications of the iOCT on surgeon decision-making. Long-term patient outcomes is currently being collected and additional data will be helpful in the future to better understand the role of iOCT. This report also focuses on a single integrated iOCT system. One-year enrollment for the other DISCOVER systems is still underway and that data is expected later this year. Currently, randomized masked controlled studies are also being planned to provide more definitive answers to the overall value of iOCT in patient outcomes.
In this report, iOCT imaging was successfully achieved at a very high rate (99%). For lamellar keratoplasty procedures, iOCT was reported to alter the surgical procedure in 33% of cases. The most common reason for iOCT changing the approach to the surgical procedure was discordance between surgeon perception of graft adherence and the objective iOCT information. Similarly for posterior segment membrane peeling procedures, iOCT provided new information to the surgeon in 19% of cases that was not in agreement with surgeon impression. In these cases, the most common reason for altering a surgical procedure was related to completeness of peel. These numbers are similar to other reported studies. In the PIONEER study, surgeons reported that in lamellar keratoplasty cases, iOCT definitively changed the surgical approach in 9% of cases.1 In retinal membrane peeling procedures, iOCT changed surgical approach in 13% of cases.1 Similar to DISCOVER, these were cases where the subjective interpretation of the en face view by the surgeon was discordant with the objective iOCT information.1
Generally, surgeons reported immediate feedback related to tissue anatomy changes to be most valuable (e.g., completeness of membrane peel, graft adherence). True “real-time” visualization of surgical maneuvers was less commonly reported as critical. Select cases such as viscodissection and choroidal biopsies appeared to be particularly helpful with real-time feedback. One current major limitation of real-time visualization is the lack of OCT-compatible instrumentation.11-13 Metallic instruments result in significant shadowing with suboptimal light scattering properties for OCT visualization. Improvement in OCT-compatible instrumentation may advance the field even further.11-13 Although the systems in DISCOVER represent a significant iterative step forward related to integrative technologies, deficits remain in the technology for true seamless integration. Current deficits include automated OCT aiming/tracking, optimizing heads-up display, instrument-depth tracking, and software analysis for iOCT alterations, in addition to OCT-compatible instrumentation.11,18,19 Optimizing heads-up display for maximizing surgeon feedback while minimizing distraction will be important.11 Significant advances have been achieved with iOCT software analysis packages, including assessment of interface fluid and volumetric pathology analysis for features, such as macular hole.5,18-20
The definitive role for iOCT continues to emerge. Research from the PIONEER study has shown that minimizing interface fluid in DSAEK procedure may reduce postoperative interface haze.16 Additionally, alterations in the outer retina (ellipsoid zone-retinal pigment epithelium height) may be important findings understanding the complete architectural normalization following MH repair.20 An exciting potential emerging role for iOCT is in targeted therapeutic delivery with image-guided tissue placement and objective volumetric measurement may have a critical role in the future in regenerative medicine and gene-therapy.
The 1-year results of the RESCAN portion of the DISCOVER study provide additional evidence for the feasibility, utility and potential clinical implications of microscope-integrated iOCT. As additional barriers to seamless integration are cleared, such as software analysis and automated tracking, the ease and role for iOCT in ophthalmic surgery may continue to expand. Ongoing research in long-term patient outcomes related to the PIONEER, DISCOVER and other ongoing studies will continue to add to our knowledge base and improve our understanding of how this may add value to our surgical procedures.
Supplementary Material
Acknowledgements
A. Funding and support: NIH/NEI K23-EY022947-01A1 (JPE); Ohio Department of Development TECH-13-059 (JPE, WJD, SKS); Research to Prevent Blindness (Institutional Cole Grant); All funding sources had no input into the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
B. Financial Disclosures: JPE: Bioptigen (consultant, intellectual property), Thrombogenics (consultant, research funding), Synergetics (intellectual property), Genentech (research funding), Leica (consultant), Zeiss (consultant), Alcon (consultant); JG: None; WJD: Zeiss (research funding); PKK: Zeiss (consultant), Topcon (consultant), Alcon (consultant), Novartis (consultant), Bausch and Lomb (consultant); RPS: Zeiss (consultant); RG: None; JE: None; SKS: Bausch and Lomb (consultant, research grant); Bioptigen (intellectual property); Allergan (research grant); Synergetics (intellectual property); Leica (consultant), Carl Zeiss Meditec (consultant)
Footnotes
C. Author Contributions: Design of the study (JPE, JG WJD, PKK, SKS); Conduct of the study (All authors); Data collection (All authors); Data management (JPE, SKS); Data analysis (All authors); Data Interpretation (All authors); Preparation of the manuscript (JPE); Review and approval of the manuscript (All authors); Provision of patients (All authors). The principal investigator (JPE) had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis
References
- 1.Ehlers JP, Dupps WJ, Kaiser PK, et al. The Prospective Intraoperative and Perioperative Ophthalmic ImagiNg With Optical CoherEncE TomogRaphy (PIONEER) Study: 2-Year Results. American journal of ophthalmology. 2014;158(5):999–1007. doi: 10.1016/j.ajo.2014.07.034. e1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ehlers JP, Kaiser PK, Srivastava SK. Intraoperative optical coherence tomography using the RESCAN 700: preliminary results from the DISCOVER study. The British journal of ophthalmology. 2014;98(10):1329–1332. doi: 10.1136/bjophthalmol-2014-305294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ehlers JP, Ohr MP, Kaiser PK, Srivastava SK. Novel microarchitectural dynamics in rhegmatogenous retinal detachments identified with intraoperative optical coherence tomography. Retina. 2013;33(7):1428–1434. doi: 10.1097/IAE.0b013e31828396b7. [DOI] [PubMed] [Google Scholar]
- 4.Ehlers JP, Tam T, Kaiser PK, Martin DF, Smith GM, Srivastava SK. Utility of intraoperative optical coherence tomography during vitrectomy surgery for vitreomacular traction syndrome. Retina. 2014;34(7):1341–1346. doi: 10.1097/IAE.0000000000000123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ehlers JP, Xu D, Kaiser PK, Singh RP, Srivastava SK. Intrasurgical dynamics of macular hole surgery: an assessment of surgery-induced ultrastructural alterations with intraoperative optical coherence tomography. Retina. 2014;34(2):213–221. doi: 10.1097/IAE.0b013e318297daf3. [DOI] [PubMed] [Google Scholar]
- 6.Binder S, Falkner-Radler CI, Hauger C, Matz H, Glittenberg C. Feasibility of intrasurgical spectral-domain optical coherence tomography. Retina. 2011;31(7):1332–1336. doi: 10.1097/IAE.0b013e3182019c18. [DOI] [PubMed] [Google Scholar]
- 7.Pichi F, Alkabes M, Nucci P, Ciardella AP. Intraoperative SD-OCT in macular surgery. Ophthalmic surgery, lasers & imaging : the official journal of the International Society for Imaging in the Eye. 2012;43(6 Suppl):S54–60. doi: 10.3928/15428877-20121001-08. [DOI] [PubMed] [Google Scholar]
- 8.Ray R, Baranano DE, Fortun JA, et al. Intraoperative Microscope-Mounted Spectral Domain Optical Coherence Tomography for Evaluation of Retinal Anatomy during Macular Surgery. Ophthalmology. 2011;118(11):2212–2217. doi: 10.1016/j.ophtha.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 9.Steven P, Le Blanc C, Velten K, et al. Optimizing descemet membrane endothelial keratoplasty using intraoperative optical coherence tomography. JAMA ophthalmology. 2013;131(9):1135–1142. doi: 10.1001/jamaophthalmol.2013.4672. [DOI] [PubMed] [Google Scholar]
- 10.Scorcia V, Busin M, Lucisano A, Beltz J, Carta A, Scorcia G. Anterior segment optical coherence tomography-guided big-bubble technique. Ophthalmology. 2013;120(3):471–476. doi: 10.1016/j.ophtha.2012.08.041. [DOI] [PubMed] [Google Scholar]
- 11.Ehlers JP, Srivastava SK, Feiler D, Noonan AI, Rollins AM, Tao YK. Integrative advances for OCT-guided ophthalmic surgery and intraoperative OCT: microscope integration, surgical instrumentation, and heads-up display surgeon feedback. PloS one. 2014;9(8):e105224. doi: 10.1371/journal.pone.0105224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ehlers JP, Tao YK, Farsiu S, Maldonado R, Izatt JA, Toth CA. Integration of a spectral domain optical coherence tomography system into a surgical microscope for intraoperative imaging. Investigative ophthalmology & visual science. 2011;52(6):3153–3159. doi: 10.1167/iovs.10-6720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ehlers JP, Tao YK, Srivastava SK. The value of intraoperative optical coherence tomography imaging in vitreoretinal surgery. Current opinion in ophthalmology. 2014;25(3):221–227. doi: 10.1097/ICU.0000000000000044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hahn P, Migacz J, O'Donnell R, et al. Preclinical evaluation and intraoperative human retinal imaging with a high-resolution microscope-integrated spectral domain optical coherence tomography device. Retina. 2013;33(7):1328–1337. doi: 10.1097/IAE.0b013e3182831293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dayani PN, Maldonado R, Farsiu S, Toth CA. Intraoperative use of handheld spectral domain optical coherence tomography imaging in macular surgery. Retina. 2009;29(10):1457–1468. doi: 10.1097/IAE.0b013e3181b266bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Juthani VV, Goshe JM, Srivastava SK, Ehlers JP. Association between transient interface fluid on intraoperative OCT and textural interface opacity after DSAEK surgery in the PIONEER study. Cornea. 2014;33(9):887–892. doi: 10.1097/ICO.0000000000000209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tao YK, Srivastava SK, Ehlers JP. Microscope-integrated intraoperative OCT with electrically tunable focus and heads-up display for imaging of ophthalmic surgical maneuvers. Biomedical optics express. 2014;5(6):1877–1885. doi: 10.1364/BOE.5.001877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu D, Dupps WJ, Srivastava S, Ehlers JP. Automated volumetric analysis of interface fluid in descemet stripping automated endothelial keratoplasty utilizing intraoperative optical coherence tomography. Investigative ophthalmology & visual science. 2014;55(9):5610–5615. doi: 10.1167/iovs.14-14346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu D, Yuan A, Kaiser PK, et al. A novel segmentation algorithm for volumetric analysis of macular hole boundaries identified with optical coherence tomography. Investigative ophthalmology & visual science. 2013;54(1):163–169. doi: 10.1167/iovs.12-10246. [DOI] [PubMed] [Google Scholar]
- 20.Ehlers JP, Itoh Y, Xu L, Kaiser PK, Singh RP, Srivastava SK. Factors Associated with Persistent Subfoveal Fluid and Complete Macular Hole Closure in the PIONEER Study. Investigative ophthalmology & visual science. 2014;56(2):1141–6. doi: 10.1167/iovs.14-15765. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.