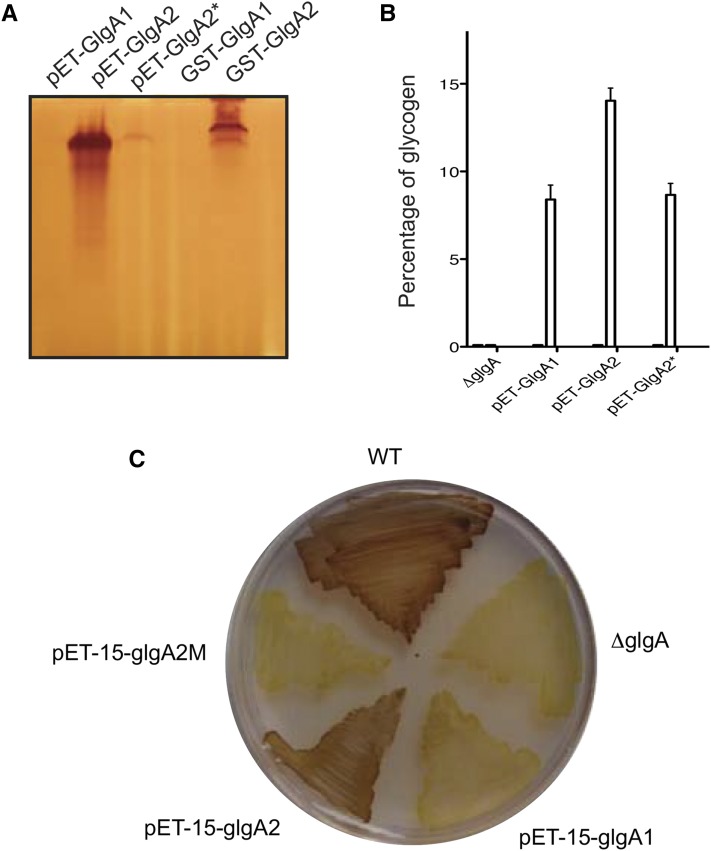
Figure 6.
Complementation experiments and recombinant protein expression of GlgA1 and GlgA2 of Cyanobacterium sp. CLg1. A, Recombinant protein expression of glutathione S-transferase (GST)-tagged glycogen/starch synthase GlgA1 and GlgA2 (pGEX-glgA1 and pGEX-glgA2) and untagged proteins (pET-glgA1, pET-glgA2, and pET-glgA2*) were expressed in the ΔglgA mutant stain (JW3392-1) of E. coli. Crude extracts were loaded on native PAGE gels containing glycogen. After migration, the gel was incubated overnight in the incubation buffer containing 3 mm ADP-Glc. Provided that their glucan products are sufficiently long to form helices that stably trap iodine, glycogen/starch synthase activities may be revealed as black bands staining on an orange background after soaking the native gel in iodine solution. B, Restoration of glycogen synthesis in the presence of mannitol or maltose as a carbon source in the ΔglgA mutant expressing untagged protein GlgA1, GlgA2, and GlgA2* (pET-glgA1, pET-glgA2, and pET-glgA2*). The amount of glycogen for each strain was determined by the amyloglucosidase assay. The glycogen measured in the wild-type strain was used as a reference (70 ± 20 μg glycogen mg−1 protein). The results are expressed as percentages of glycogen amounts accumulated by our wild-type reference. C, Iodine staining of the wild type strain (WT) and the ΔglgA strains of E. coli expressing the recombinant proteins GlgA1, GlgA2, and GlgA2* cultured in solid synthetic medium supplemented with 50 mm maltose.