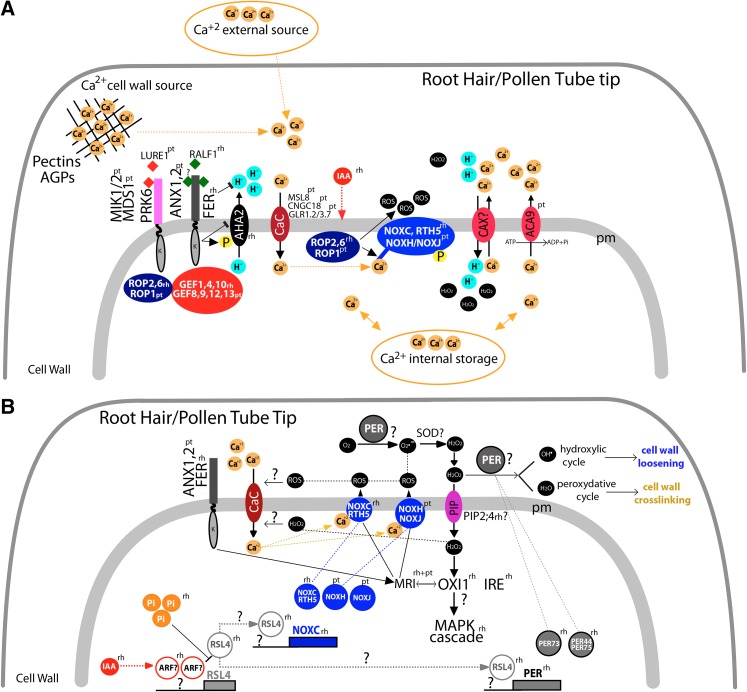
Figure 1.
Proposed scheme to describe how ROS are interconnected with Ca2+-pH signaling components in polar growing cells. A, Ca2+ ions are taken up by the cell from external sources or stored in the cell wall and released by changes in apopH controlled mainly by plasma membrane AHA. In addition, Ca2+ can be stored in subcellular compartments (e.g. vacuoles and ER-Golgi) and released into the cytosol. Still poorly characterized, plasma membrane CaCs transport free Ca2+ ions from the apoplast into the cytoplasm (Table I). To maintain physiologically low concentrations of cytCa2+, ACAs transport Ca2+ ions back to the apoplast. In addition, the H+/Ca2+ antiporter activity of CAX translocates Ca2+ back to the apoplast and, simultaneously, imports H+ into the cytoplasm. apoROS production is catalyzed by the NOX proteins, NOXC and RTH5 (in maize) in root hairs and NOXH/NOXJ in pollen tubes. These NOX proteins are regulated by complex partners, including Ca2+ ions, ROPs, and kinases. NOX produces apoplastic ion superoxide (O2−), which mostly is converted by superoxide dismutases (SODs) to H2O2. In root cells, the CrRLK1L kinase FER, which contains a malectin-like extracellular domain, binds to RALF1 and inhibits growth. In growing root hairs, FER forms a complex with the guanine nucleotide-exchange factor ROP-GEF1, and possibly with other ROP-GEFs (such as ROP-GEF4 or ROP-GEF10), to recruit and then activate the plant RHO GTPase ROP2. In pollen tubes, ANX1 and ANX2 are the CrRLK1L proteins that regulate ROS-linked cell growth. In addition, pollen tube guidance to the ovule relies on two groups of Leu-rich receptor-like kinases (MDS1-MIK1/2 and PRK6) binding to the Cys-rich peptide LURE1 (see text for details) and downstream ROPGEFs (ROPGEF8, ROPGEF9, ROPGEF12, and ROPGEF13) and ROP1 (at least demonstrated for PRK6). Then, ROP1 possibly targets the N-terminal domain of NOXH/NOXJ to trigger ROS production. In root hairs, spatially active ROP2 triggers ROS production, since it is able to bind directly to the N terminus of NOXC and enhance its enzymatic activity. Auxin (as indole acetic acid [IAA]) enhances ROP2 activity to generate ROS by triggering NOXC activation. B, In root hairs, high auxin levels (IAA), possibly attained through the activation of unknown ARF proteins, up-regulate the expression of the transcription factor basic helix-loop-helix RSL4 (in addition to activating ROP2) and promote ROS production. It is proposed here that RSL4 controls (directly or indirectly) the transcription of NOXC/RTH5 and NOXH/NOXJ as well as secreted type III peroxidases (PER) in root hairs. By contrast, high levels of Pi act as a repressor of RSL4 expression. High concentrations of apoROS enhance or reduce the stiffness of the cell wall, thereby either promoting or restricting cellular extension. PER uses H2O2 as an oxidant to convert cell wall phenolic compounds and structural proteins into free radicals that can subsequently come together to form covalent linkages (in the peroxidative cycle), thus restricting growth. Also, apoH2O2 and oxygen (O2) can be used to generate hydroxyl radicals (in the hydroxylic cycle), including •OH, which mediates the nonenzymatic cleavage of polysaccharides. This would induce transient relaxation of the cell wall, allowing tip growth. ROS also could promote Ca2+ transport by triggering the activation of CaCs (Table I) by unknown mechanisms, possibly in the apoplast, cytoplasm, or on both sides. Not all ROS remains in the apoplast, since some of the H2O2 may be transported back to the cytoplasm by aquaporins (plasma membrane intrinsic proteins [PIPs]) and trigger a signal cascade response (e.g. OXI1-mediated MAPK cascade). OXI1, an AGC2 kinase, could activate MARIS (MRI), a receptor-like cytoplasmic kinase present in growing root hairs and pollen tubes. MRI would be a downstream component of FER in root hairs and of ANX1/ANX2 in pollen tubes. Other root hair-specific AGC kinases in the VIII subfamily are IRE and AGC2-1. Solid arrows indicate a signaling pathway, a downstream step, or transport across the plasma membrane; solid lines indicate a close relationship between proteins or ions. P, Phosphorylated site; pm, plasma membrane; pt, pollen tubes; rh, root hairs.