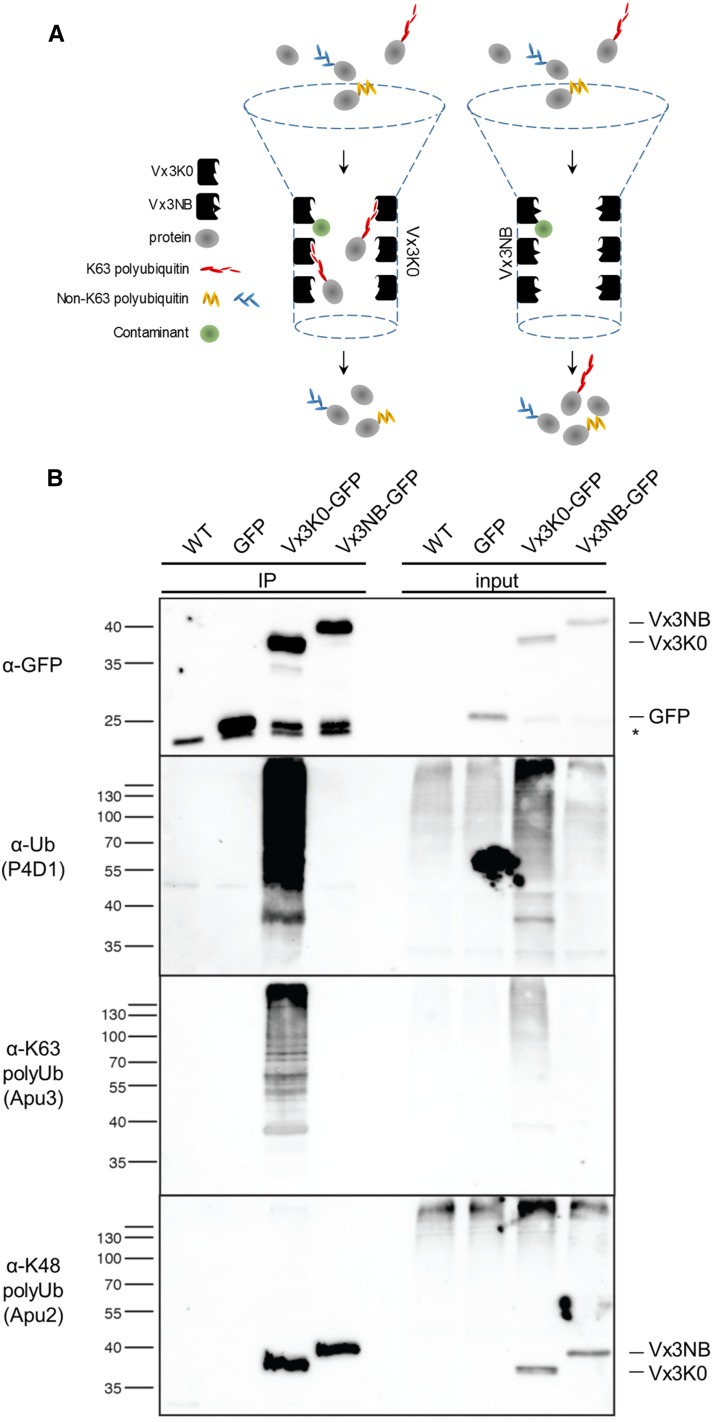
Figure 2.
Biochemical characterization of the Vx3K0 and Vx3NB sensors. A, Scheme representing the principle of K63 polyubiquitinated protein purification using Vx3K0 (left) and the nonbinding Vx3NB as a control (right). B, In vivo characterization of purified proteins using sensor-based immunoprecipitation of proteins carrying K63 polyubiquitin chains. Immunoprecipitation (IP) was performed using anti-GFP antibodies on RIPA buffer-solubilized protein extracts from wild-type (WT) and monoinsertional homozygous plants expressing free GFP, Vx3K0-HA-GFP, and Vx3NB-HA-GFP and subjected to immunoblotting with anti-GFP, anti-ubiquitin P4D1, anti-K63 polyubiquitin Apu3, and anti-K48 polyubiquitin Apu2 antibodies. The membrane was probed with the respective antibodies and stripped in the following order: anti-GFP, anti-K63 polyubiquitin (Apu3), anti-K48 polyubiquitin (Apu2), and anti-ubiquitin (P4D1) antibodies. The sizes of marker proteins in kD are indicated next to the respective blots. The asterisk indicates a nonspecific band recognized by the anti-GFP antibodies. Note that both Vx3K0 and Vx3NB sensors show partial cleavage, as attested by the presence of a signal migrating at the size of free GFP.