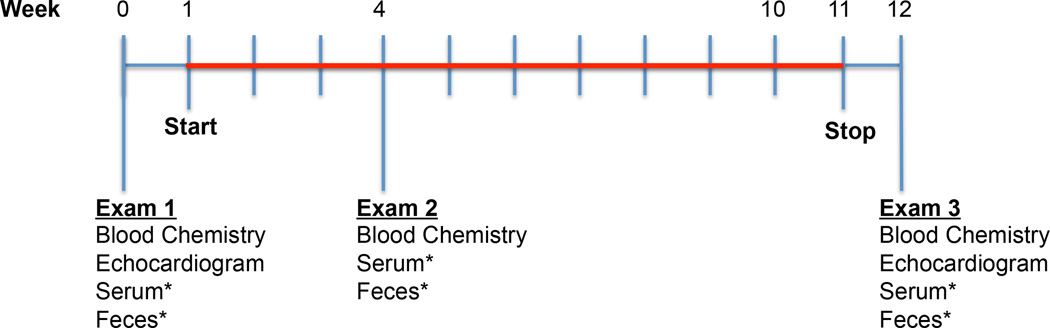
Figure 3. Design of the current short-term rapamycin intervention trial.
Dogs must weigh at least 40 pounds and be at least 6 years old at time of entry into the study. If no significant pre-existing health conditions are detected at the first exam, dogs are randomized into either placebo or one of the rapamycin treatment groups. Red indicates the 10 week period during which the dogs receive either rapamycin or placebo. Dogs receive the same generic rapamycin (sirolimus) pill that is provided to human patients. *Serum and feces are collected at each appointment for future metabolomic and microbiome analyses and for quantitation of circulating rapamycin levels.