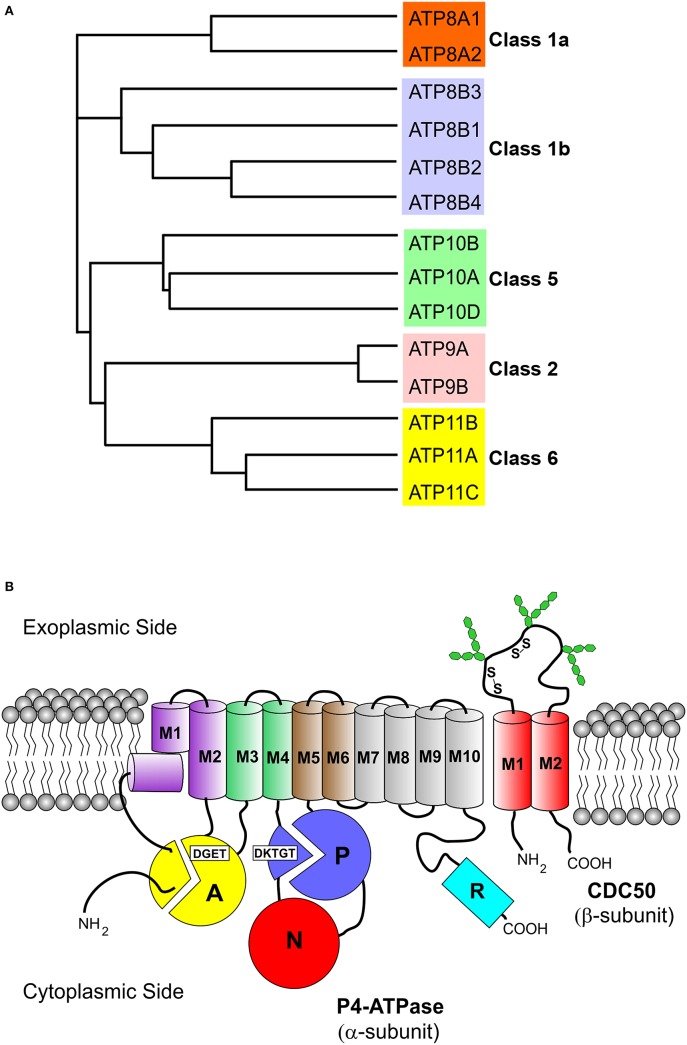
Figure 1.
Phylogenetic analysis and membrane topology of P4-ATPases. (A) The sequences of the 14 human P4-ATPases were aligned using the Clustal Omega multi-sequence alignment program for generation of the phylogenetic tree. Protein sequences are from the following accession numbers: ATP8A1 (Q9Y2Q0); ATP8A2 (Q9NTI2); ATP8B1 (O43520); ATP8B2 (P98198); ATP8B3 (O60423); ATP8B4 (Q8TF62); ATP9A (O75110); ATP9B (O43861); ATP10A (O60312); ATP10B (O94823); ATP10D (Q9P241); ATP11A (P98196); ATP11B (Q9Y2G3); ATP11C (Q8NB49); (B) The cytoplasmic A (“actuator”), N (nucleotide binding), and P (phosphorylation) domains of the α-subunit are shown as colored circles. Conserved motifs involved in phosphorylation (DKTGT) and dephosphorylation (DGET) are indicated for the respective domains. The 10 transmembrane helices of the α-subunit are represented by cylinders (M1 kinked), M1–M2 purple, M3–M4 green, M5–M6 brown, and M7–M10 gray. The two transmembrane helices of the β-subunit (CDC50 protein) are shown as red cylinders. Glycosylation and disulfide bridges of the exoplasmic domain of the β-subunit are also indicated. The C-terminal cytoplasmic extension of the α-subunit furthermore contains a regulatory domain shown as cyan cylinder (R domain).